Novel magnetic iron/lanthanum composite arsenic removal adsorbing material and preparation method thereof
A composite material and composite adsorption technology, which is used in chemical instruments and methods, adsorption water/sewage treatment, alkali metal compounds, etc. problems, to achieve good recyclability, simple elution and regeneration, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0021] Step 1, weighing medicine; According to the metering coefficient ratio 1:2:4, weigh 0.433 g lanthanum nitrate hexahydrate (La(NO 3 ) 3 ·6H 2 O), 0.5406 g ferric chloride hexahydrate (FeCl 3 ·6H 2 O) and 0.6645 g terephthalic acid (H 2 BDC).
[0022] Step 2. Dissolving the drug: put the weighed drug into a PTFE liner with a capacity of 80 mL; take 50 mL of N,N-dimethylformamide (DMF) with a graduated cylinder, and pour it into the above container , stir evenly to obtain a clear and transparent solution;
[0023] Step 3, hydrothermal reaction: Put the above-mentioned reactor with clarified solution into an electric oven, set the temperature at 150 °C, and time: 900 min; until the reaction is completed.
[0024] Filtration and drying: When the oven is lowered to room temperature, take out the reaction kettle, filter, and wash with DMF, deionized water, and ethanol three times respectively, collect samples, and dry them in a vacuum oven (parameters such as: T = 120 °C...
Embodiment example 2
[0028] Step 1, weighing medicine; According to the ratio of coordination metering coefficient 0.4:2:4, weigh 0.1732 g lanthanum nitrate hexahydrate (La(NO 3 ) 3 ·6H 2 O), 0.5406 g ferric chloride hexahydrate (FeCl 3 ·6H 2 O) and 0.6645 g terephthalic acid (H 2 BDC); according to the coordination stoichiometric coefficient ratio of 1:2:4, weigh 0.433 g lanthanum nitrate hexahydrate (La(NO 3 ) 3 ·6H 2 O), 0.5406 g ferric chloride hexahydrate (FeCl 3 ·6H 2 O) and 0.6645 g terephthalic acid (H 2 BDC); according to the coordination stoichiometric coefficient ratio of 2:2:4, weigh 0.866 g lanthanum nitrate hexahydrate (La(NO 3 ) 3 ·6H 2 O), 0.5406 g ferric chloride hexahydrate (FeCl 3 ·6H 2 O) and 0.6645 g terephthalic acid (H 2 BDC).
[0029] Step 2. Dissolving the drug: put the weighed drug into three 80 mL polytetrafluoroethylene liners; take 50 mL of N,N-dimethylformamide (DMF) with a measuring cylinder, pour into the above container and stirred vigorously with a...
Embodiment example 3
[0035] The iron / lanthanum composite material synthesized in Case 1 was used to simulate the removal of As(V) in water. The detailed process is as follows:
[0036] Step 1. Prepare a series of As(V) solutions with gradient settings such as: 2.5 mg / L, 5 mg / L, 10 mg / L, 25 mg / L, 50 mg / L, 75 mg / L, 100 mg / L L, 150 mg / L and 200 mg / L. Use 0.1 mol / L hydrochloric acid to adjust the above series of solutions to pH = 7.0±0.2, set aside.
[0037] Step 2, the dosage is controlled as: 0.25 g / L, and the shaker parameters are set as: 200 rpm, 25 °C; put the series of solutions in step 1 into the shaker, and shake at constant temperature for 24 h.
[0038] Step 3: Sampling 5 mL with a disposable syringe, filtered through a 0.22 μm aqueous membrane, and analyzed and quantified by inductively coupled plasma-spectroscopy (ICP-OES).
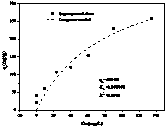
[0039] image 3 It is the adsorption isotherm of iron / lanthanum composite material, and the maximum adsorption capacity is 410mg / g simulated by Langmuir model.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com