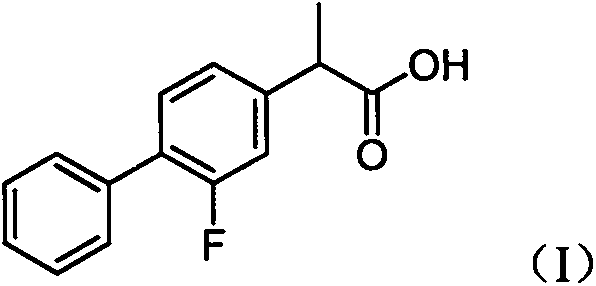
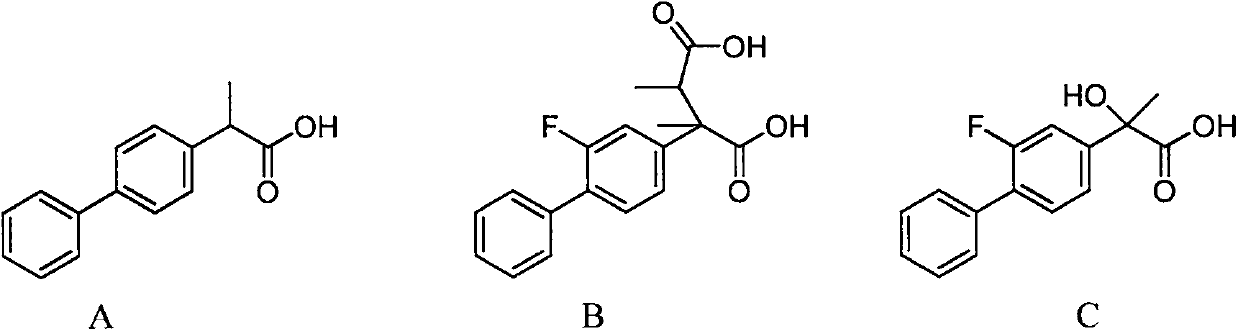
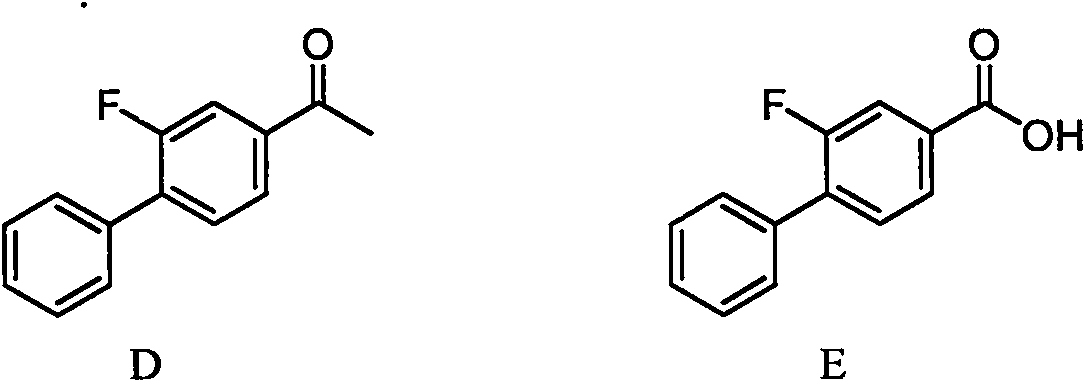
Preparation method of high-purity flurbiprofen known impurities
A flurbiprofen and impurity technology, which is applied in the preparation of organic compounds, carboxylates, carboxylates, etc., can solve problems such as no preparation impurities, and achieve improved drug safety, easy operation, and purity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Magnesium powder (2.55g, 0.105mol) was added to the reaction flask, under nitrogen protection and stirring, a solution of 4-bromo-2-fluorobiphenyl (25.1g, 0.1mol) in anhydrous tetrahydrofuran (125ml) was added dropwise, and the temperature was controlled at 20 ~35°C, after the dropwise addition was completed, the temperature was raised to reflux (65°C) for 2 hours, and the reaction was monitored by TLC; the temperature was lowered to room temperature under the protection of nitrogen, and 2-bromo-2,3-dimethylsuccinate sodium (26.9g, 0.1mol), the addition was completed, and the temperature was raised to 65°C for 2 hours. After the reaction was completed, the solvent was evaporated under reduced pressure, and toluene (125ml) and dilute hydrochloric acid (125ml) were added, stirred and heated to dissolve, then left to stand, the organic phase was separated, and the aqueous phase was used Extract once with toluene (50ml), combine the organic phases, wash with purified water u...
Embodiment 2
[0039] Take 10g of the crude product of 2-(2-fluoro-4-biphenyl)-2,3-dimethylsuccinic acid, 70ml of methanol and water (1:1, v / v), heat and dissolve under stirring, add activated carbon 0.2g, reflux and decolorize for 0.5h, heat filter, stir the filtrate to cool down at 0-10°C, crystallize for 1h, filter, and dry the filter cake under vacuum at 45°C to get 2-(2-fluoro-4-biphenyl)- The refined product of 2,3-dimethylsuccinic acid was 7.72g, the HPLC purity was 99.1%, and the yield was 77.2%.
Embodiment 3
[0041] Take 10g of the crude product of 2-(2-fluoro-4-biphenyl)-2,3-dimethylsuccinic acid, 50ml of ethanol and water (2:1, v / v), heat and dissolve under stirring, add activated carbon 0.5g, decolorized under reflux for 0.5h, hot filtered, the filtrate was stirred and cooled down to 0-10°C, crystallized for 1h, filtered, and the filter cake was dried under vacuum at 45°C to obtain 2-(2-fluoro-4-biphenyl)- The refined product of 2,3-dimethylsuccinic acid was 7.55g, the HPLC purity was 99.4%, and the yield was 75.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com