Potassium sodium dehydroandrographolide succinate enteric-coated preparation and preparation method
A technology of Yanhuning intestinal and enteric-coated preparations, which is applied in antiviral agents, pharmaceutical formulas, medical preparations of non-active ingredients, etc., and can solve allergic reactions or pyrogen-like reactions, patients' psychological and physiological resistance, and production process requirements High-level problems, to achieve the effect of protecting active ingredients, increasing the difficulty of quality control, and reducing allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of refined Yanhuning
[0035] Use the commercially available Chuanhuning, after lipidation reaction with sodium sulfite, then directly synthesize Yanhuning with one of sodium bicarbonate and potassium bicarbonate or according to a specific ratio, so as to reduce repeated drying, dissolving, heating and other processes. The specific preparation method is as follows: taking Chuanhuning as raw material, adding anhydrous sodium sulfite and 90% ethanol into an extraction tank, simultaneously passing nitrogen protection, stirring and mixing, heating in a water bath, and controlling the temperature at 60°C. After the raw materials are dissolved, stop heating and slowly add 19% KHCO 3 and 10% Na 2 CO 3 Mix solution (mixed according to mass ratio 1:1), produce CO 2 gas, crystals are precipitated in the reaction solution, and the CO 2 After the production is completed, heat it in a water bath at a temperature of about 60°C until it dissolves, then add a...
Embodiment 2
[0036] Embodiment 2: preferred isolation layer material
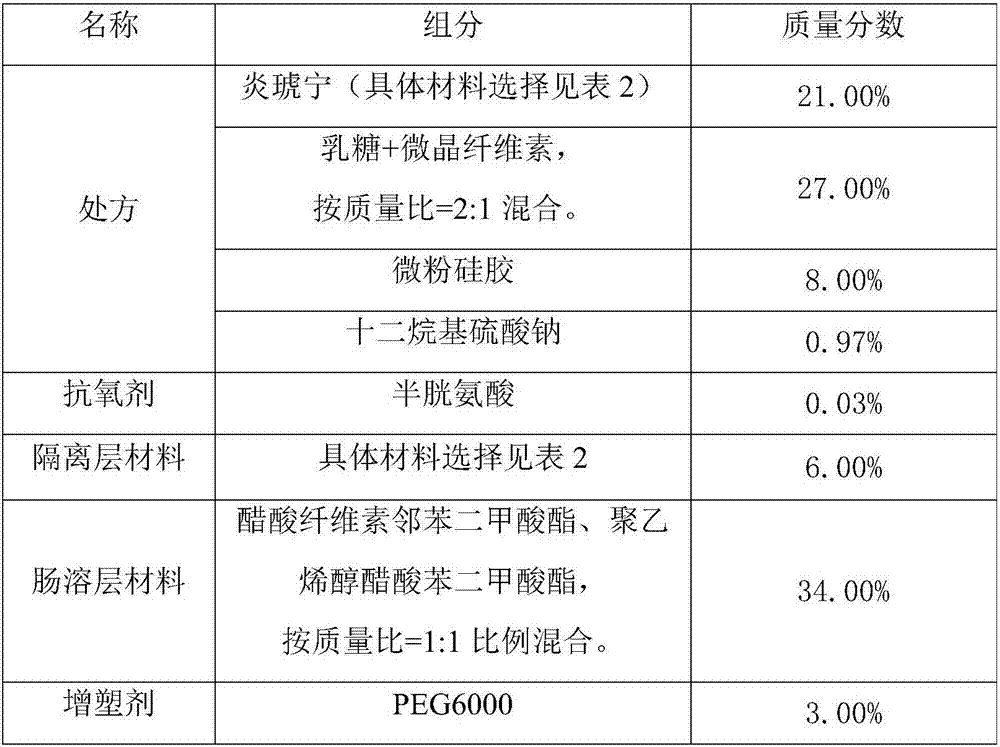
[0037] (1) The composition of Yanhuning enteric-coated preparation is shown in Table 1.
[0038] Table 1 The composition of Yanhuning enteric-coated preparation
[0039]
[0040] When preparing the mixture of lactose and microcrystalline cellulose, the mass ratio of 2:1 is prepared at one time, so for the convenience of operation, the ratio of 2:1 is selected throughout the text. In practice, lactose: microcrystalline cellulose = 1:1 to 3:1 is acceptable. If this ratio is exceeded and the lactose is too high, it may cause difficulty in forming pills and tablets in the later stage.
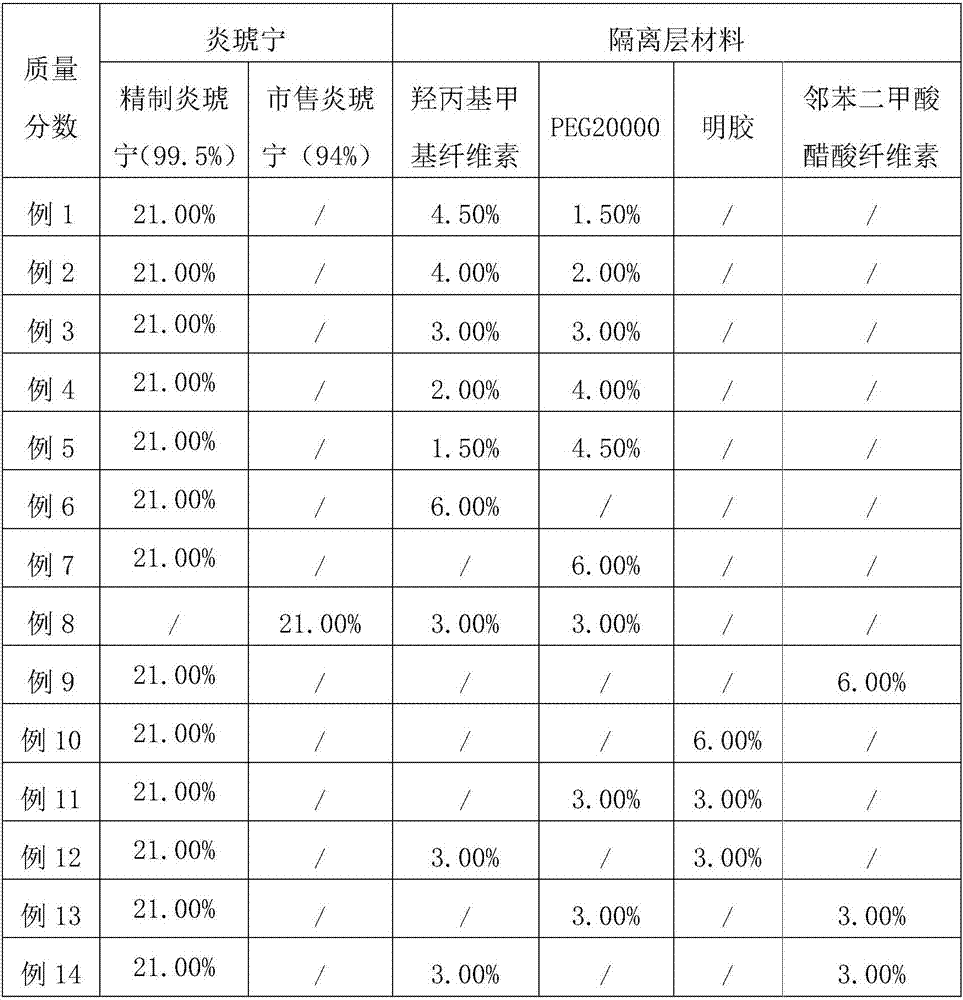
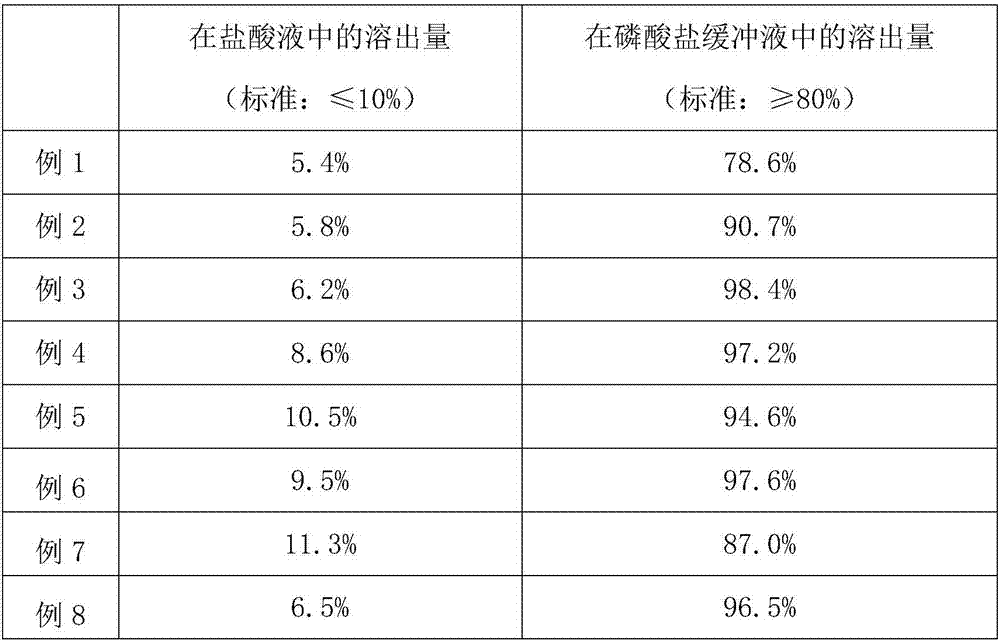
[0041] (2) Based on the mass fractions of the prescriptions in Table 1, respectively select Yanhuning with different precisions, and different materials and different mass ratios of the isolation layer materials, and carry out the screening of Yanhuning and isolation layer materials. The specific selection is as follows: Table 2 shows....
Embodiment 3
[0056] Example 3: Recipe and preparation method of Yanhuning enteric-coated preparation
[0057] (1) The composition of Yanhuning enteric-coated preparation is shown in Table 4.
[0058] Table 4 The composition of Yanhuning enteric-coated preparation
[0059]
[0060]
[0061] (2) The refined Yanhuning was prepared according to the method of Example 1, and the precision was 99.5%.
[0062] (3) Isolation layer coating. With purified water or 80% ethanol aqueous solution, the isolation layer material is mixed, and it is prepared as a solution with a solid content of 5%, and then the antioxidant cysteine is added to form a solution with a solid content of 5% (in order to control product quality, The solid content needs to be controlled at 3%~15%), stir evenly with an air pump mixer. Pass Yanhuning, lactose, microcrystalline cellulose, and micropowder silica gel through a 200-mesh sieve, and put them into a high-speed centrifugal granulator coating machine to directly c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com