Phenolic hydroxyl-containing amino acid-N-thiocarboxylic anhydride monomer and synthesis and polymerization method thereof
A technology of phenolic hydroxyl amino acid and thiocarboxylic anhydride, which is applied in the field of polymer synthesis, can solve problems such as direct synthesis of polyamino acids that have not yet been seen, and achieve good biocompatibility and biodegradability, cheap and easily available raw materials, and high initiation activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
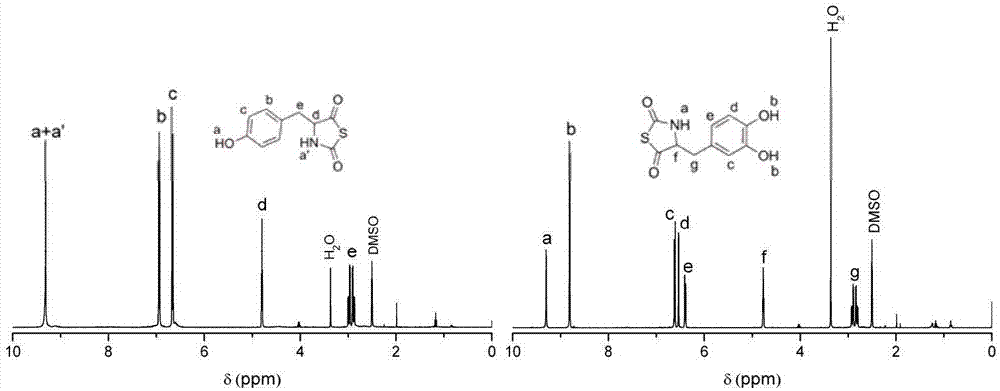
[0032] Add 36.2g (0.20mol) of tyrosine, 32.0g (0.80mol) of sodium hydroxide, 36.0g (0.20mol) of S-ethoxythiocarbonylthioglycolic acid (XAA) into the reaction flask, and deionize with 150mL Dissolved in water, stirred at room temperature for 72 hours to synthesize 31.3 g of N-ethoxycarbonyl substituted tyrosine (0.12 mol, yield 58.2%). The latter was mixed with PBr in ethyl acetate 3 9.63 g of Tyr-NTA monomer (0.043 mol, yield of 35.7%) was obtained through reaction to form a ring.
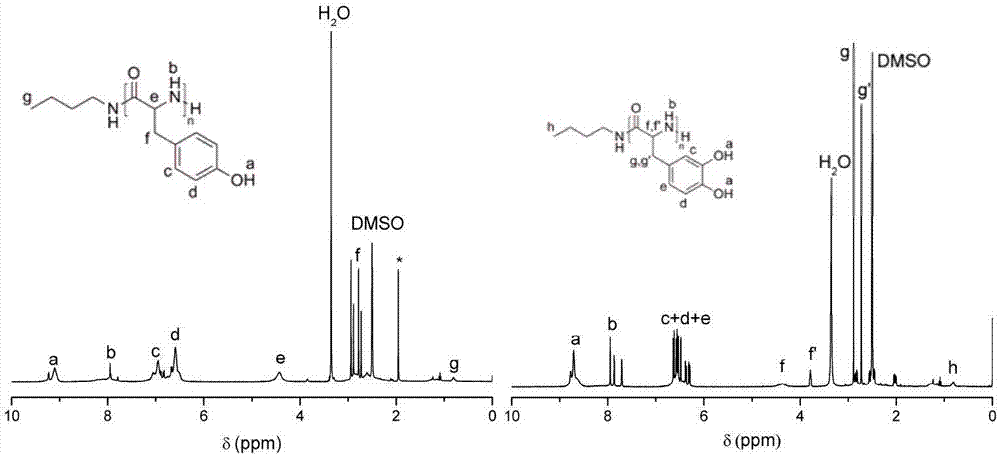
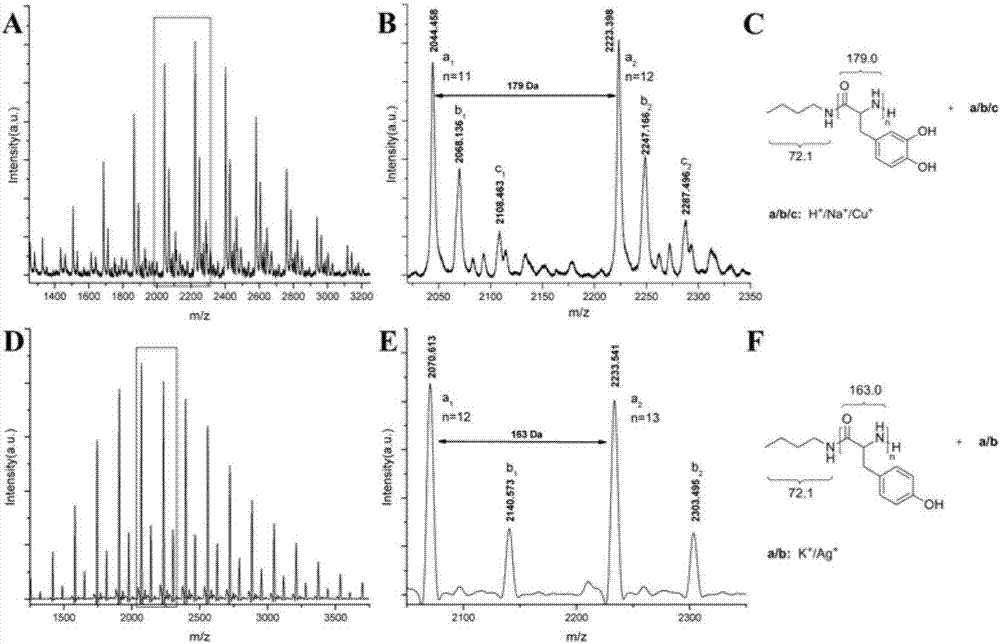
[0033] Add 0.223g (1.00mmol) of Tyr-NTA to the reaction flask, dissolve it with 2.0mL tetrahydrofuran (THF), then add 0.12mL of n-butylamine in THF (0.170mol / L, 0.020mmol), Tyr-NTA and n-butylamine The molar ratio of butylamine is 50:1, sealed and placed in an oil bath at 40°C to react for 4 days. After the reaction, the mixture is poured into ether to precipitate, filtered, and the obtained polymer is vacuum-dried for 1 day to obtain polytyrosine , the yield was 94.2%. The number-average absolu...
Embodiment 2
[0035] Other polymerization conditions are identical with embodiment 1, difference is to initiate Tyr-NTA ring-opening polymerization with benzylamine as initiator, solvent is N-methylpyrrolidone (NMP), and the mol ratio of Tyr-NTA and benzylamine is 20: 1. React in a constant temperature bath at 80°C for 12 hours to obtain polytyrosine with a yield of 85.8%. The number average absolute molecular weight of the obtained polymer was 3.0 kDa.
Embodiment 3
[0037] Other polymerization conditions are the same as in Example 1, except that the amino-terminated polyethylene glycol (PEG-NH 2 ) triggers Tyr-NTA ring-opening polymerization, the solvent is N,N-dimethylacetamide (DMAc), Tyr-NTA and PEG-NH 2 The molar ratio of the polyamino acid is 10:1, and it is reacted in a constant temperature bath at 0° C. for 2 days, and the yield of the obtained polyamino acid is 99.0%. The number average absolute molecular weight of the obtained copolymer was 3.7 kDa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Absolute molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com