Method for synthesizing glutaric acid from alpha-ketoglutaric acid
A technology of ketoglutaric acid and glutaric acid is applied in the field of preparation of glutaric acid, which can solve the problems of not conforming to the concept of sustainable development of green chemistry, expensive raw material sources, difficult to synthesize and use in large quantities, etc. The effect of safe conditions and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] specific implementation
[0032] In order to understand the present invention, the present invention will be further described below in conjunction with embodiment: following embodiment is illustrative, not limiting, can not limit protection scope of the present invention with following embodiment.
[0033] A method for the synthesis of glutaric acid from alpha-ketoglutaric acid, the steps are as follows:
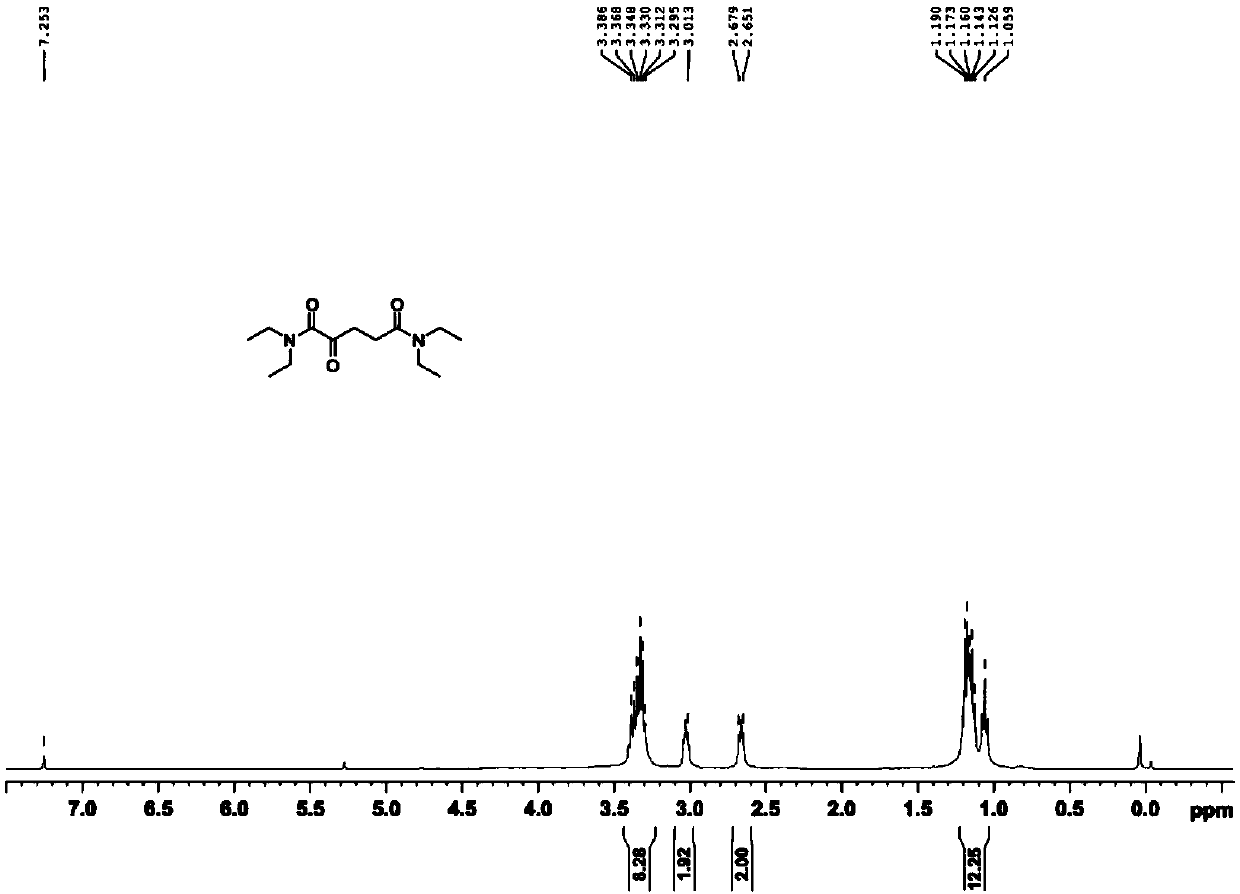
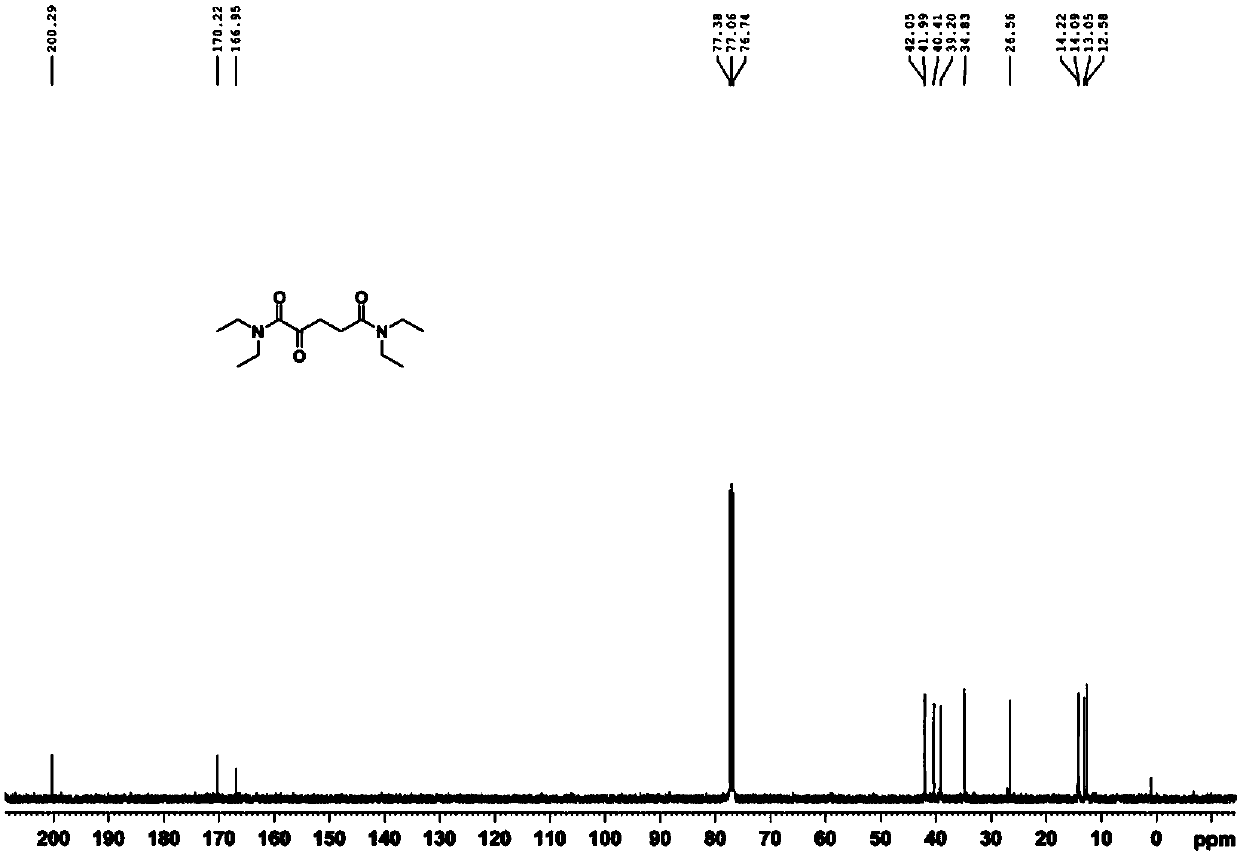
[0034] 1. Compound 1 (N 1 ,N 1 ,N 5 ,N 5 -tetraethyl-2-oxopentanediamide) synthesis.
[0035]
[0036] Add α-ketoglutaric acid (10.00g, 68.45mmol) and thionyl chloride (15.00ml, 205.34mmol) to a round bottom flask, and place the reaction mixture under reflux for one hour at 80°C. Pressure distilled to dark brown viscous oily liquid. After dissolving in tetrahydrofuran (20.00 ml), diethylamine was slowly added dropwise to the above mixture at a temperature of -10°C, and the reaction was continued overnight at room temperature with stirring. After the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com