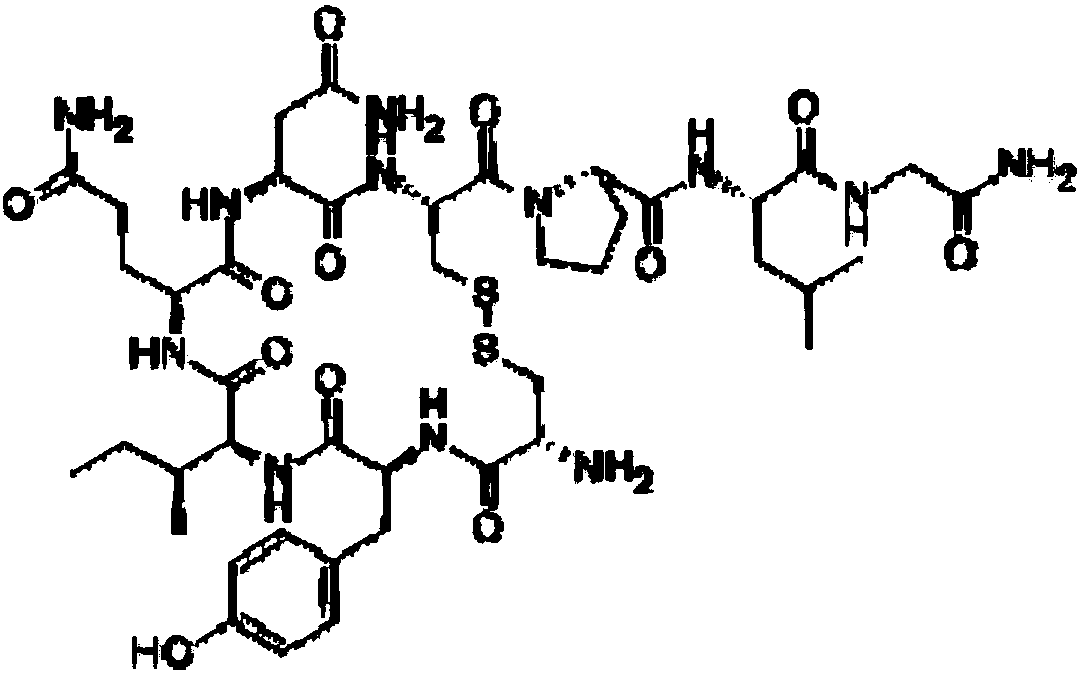
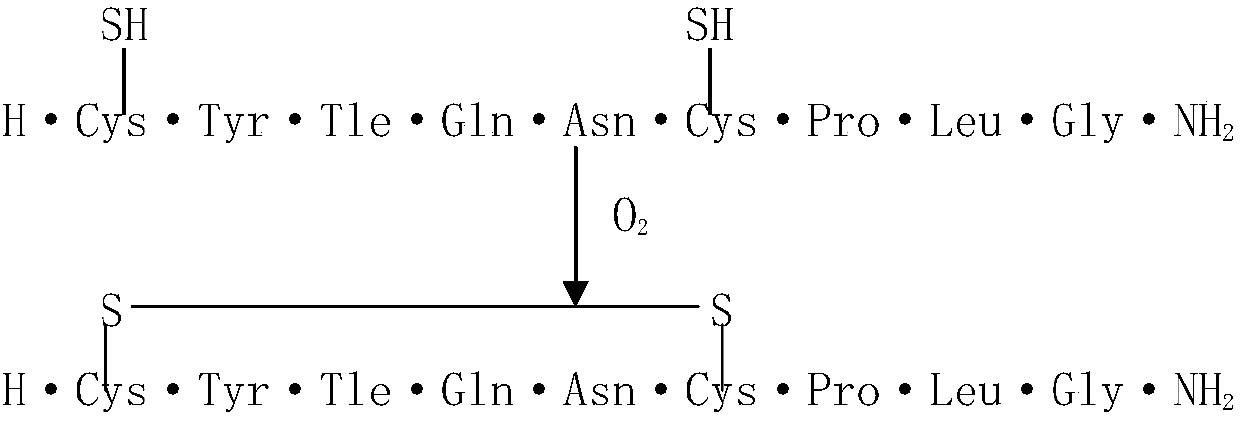
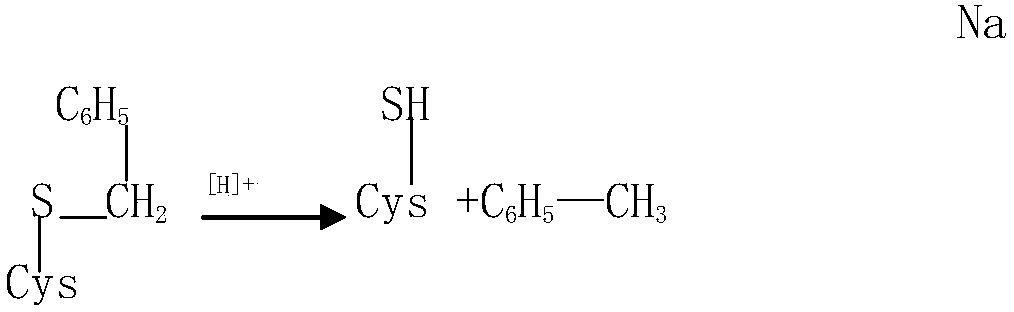Oxytocin purifying method
A purification method and oxytocin technology, applied in the field of medicine, can solve the problems of low titer and low yield, and achieve the effects of simple operation, improved product purity, and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A kind of purification method of oxytocin, the method comprises the following steps:
[0053] 1. Nonapeptide-intermediate production process
[0054] 1.1. Preparation
[0055] Pour about 600ml of liquid ammonia into a 1L three-neck round bottom flask, place the reaction flask on a magnetic stirrer, then add 16g of nonapeptide intermediate, stir to dissolve, and add an appropriate amount of ammonium chloride;
[0056] 1.2. Vacuum drying
[0057] Turn on the vacuum pump and unplug the drying tube on the reaction bottle. After the volatile ammonia gas is exhausted, put the elbow into the mouth of the bottle, and control the degree of vacuum so as not to draw out the liquid. Until the liquid is drained, the white powder on the wall of the reaction bottle falls off.
[0058] 1.3. Dissolving
[0059] Prepare 20L of 2-4% glacial acetic acid solution in a stainless steel bucket, add 800ml into the dried reaction bottle, dissolve the dry powder and clean it, pour the soluti...
Embodiment 2
[0079] A purification method of oxytocin, different from Example 1, the procedure for setting the gradient elution is shown in Table 2:
[0080] Table 2 Gradient elution program
[0081] time / min
[0082] Other steps are the same as in Example 1, and the quality detection results of the prepared oxytocin are shown in Table 4.
Embodiment 3
[0084] A purification method of oxytocin, which is different from Example 1: the procedure for setting the gradient elution is shown in Table 3:
[0085] Table 3 Gradient elution program
[0086] time / min
A%
B%
0
98
2
25
98
2
75
90
10
76
80
20
90
80
20
[0087] Other steps are the same as in Example 1, and the quality detection results of oxytocin prepared in Example 1-Example 3 are shown in Table 4.
[0088] The oxytocin quality detection that table 4 embodiment 1-embodiment 3 prepares
[0089]
[0090] The results in Table 4 prove that, under other conditions being the same, the optimal program for gradient elution is set as shown in Table 1, and the titer of oxytocin is the highest. On this basis, regardless of the increase in mobile phase A Or reduce, the potency of oxytocin obtained is significantly reduced.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com