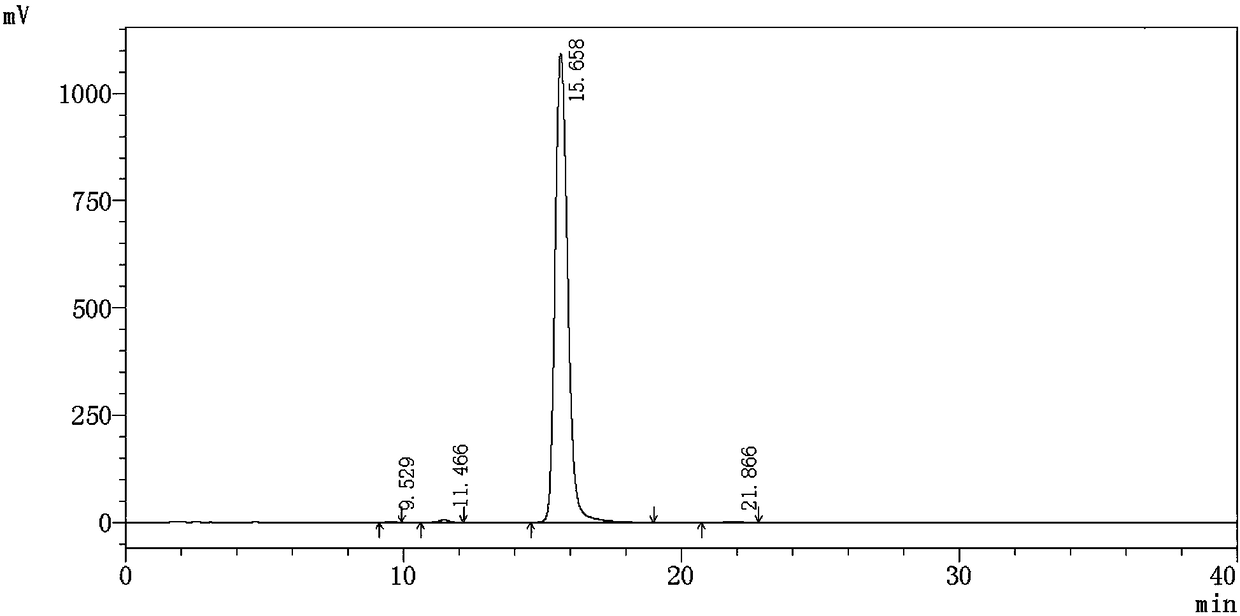
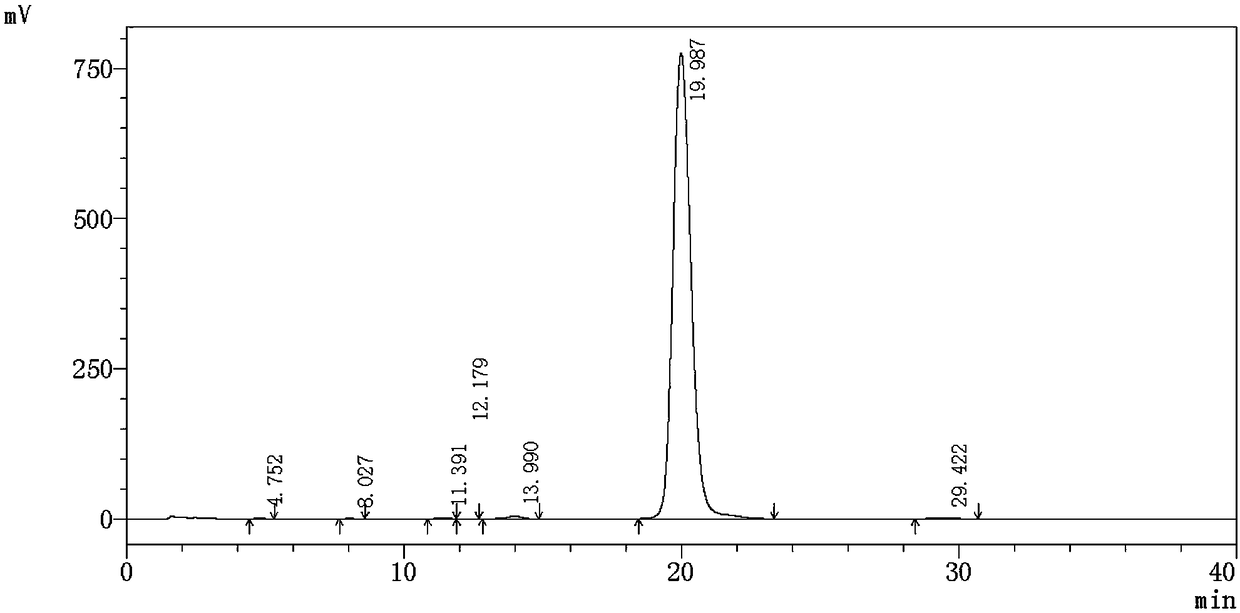
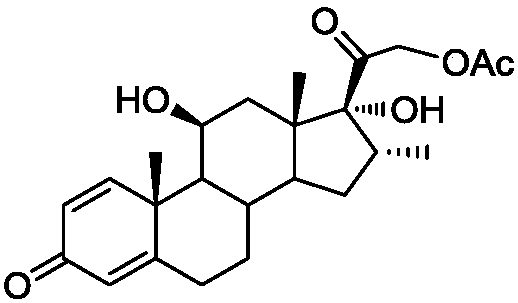
Synthesis method of alclometasone dipropionate intermediate of 11-beta hydroxypregna
A technology of hydroxypregna and dimethylformamide, which is applied in the field of synthesis of alclomethasone dipropionate intermediate 11-beta hydroxypregna, which can solve the problems of long production cycle, unsuitability for industrialization, long synthesis route, etc. problems, to achieve the effects of industrialization of production, shortening of preparation time, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1) Add 1.2kg of chromium trichloride and 21.5L of dimethylformamide into a dry and clean 50L reactor under the condition of vacuuming and nitrogen deoxidation, stir and dissolve, cool down to -5~5℃, add 1.2kg of zinc powder, Then add 1.4kg of mercaptoacetic acid dropwise, and slowly add 3.4kg of the compound of formula II dissolved in 17.2L of dimethylformamide dropwise at -10 to 0°C, and the mixed solution has been vacuumized and deoxidized by nitrogen;
[0049] 2) React at a constant temperature of -10 to 0°C for 0.5 to 1.0 hours, and monitor the disappearance of raw materials by TLC (thin layer chromatography, chloroform: acetone = 4:1);
[0050] 3) Pour the reaction solution into 164L of ice water at 0-5°C, stir for 10-20 minutes and then centrifuge, stir and dissolve the filter cake with 40.0L of tetrahydrofuran, add anhydrous sodium sulfate to dry for 1 hour, and filter;
[0051] 4) Concentrate the filtrate until the flow is cut off, add ethyl acetate to dissolve ...
Embodiment 2
[0055] 1) Add 161.5g of chromium trichloride and 3.0L of dimethylacetamide in a dry and clean 10L reaction kettle under the condition of vacuuming and nitrogen deoxidation, stir and dissolve, cool down to -5~5℃, add 157.5g of zinc powder, Add 161.5g of mercaptoacetic acid dropwise, and slowly add 600g of the compound of formula II dissolved in 2.4L of dimethylacetamide dropwise at -10 to 0°C, and the mixed solution has been evacuated and deoxidized by nitrogen;
[0056] 2) React at a constant temperature of -10 to 0°C for 0.5 to 1.0 hours, and monitor the disappearance of raw materials by TLC (thin layer chromatography, chloroform: acetone = 4:1);
[0057] 3) Pour the reaction solution into 24L of ice water at 0-5°C, stir for 10-20 minutes and then centrifuge, stir and dissolve the filter cake with 8.0L tetrahydrofuran, add anhydrous sodium sulfate to dry for 1 hour, and filter;
[0058]4) Concentrate the filtrate until the flow is cut off, add ethyl acetate to dissolve the re...
Embodiment 3
[0062] 1) Add 113.0g of chromium trichloride, 1.0L of dimethylformamide and 1.1L of acetone into a dry and clean 10L reaction kettle under the condition of vacuuming and nitrogen deoxidation, stir and dissolve, cool down to -5~5℃ and add zinc powder 110.2gg, then add 140.6g of thioglycolic acid dropwise, and slowly add 420g of the compound of formula II dissolved in 1.7L of dimethylacetamide at -10 to 0°C, and the mixed solution has been evacuated and deoxidized by nitrogen;
[0063] 2) React at a constant temperature of -10 to 0°C for 0.5 to 1.0 hours, and monitor the disappearance of raw materials by TLC (thin layer chromatography, chloroform: acetone = 4:1);
[0064] 3) Pour the reaction solution into 20L of ice water at 0-5°C, stir for 10-20 minutes and then centrifuge, stir and dissolve the filter cake with 7.0L of tetrahydrofuran, add anhydrous sodium sulfate to dry for 1 hour, and filter;
[0065] 4) Concentrate the filtrate until the flow is cut off, add ethyl acetate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com