New method for preparing epidermal defect treating drug
A technology for preparing drugs and epidermis, which is applied in the field of preparation of drugs for the treatment of epidermal defects, can solve problems that need to be developed, and achieve the effects of promoting cell proliferation, promoting wound epidermal cell proliferation, and accelerating repair and healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Acquisition and identification of exosomes
[0051] 1. The acquisition of exosomes
[0052] (1) Provide mesenchymal stem cells
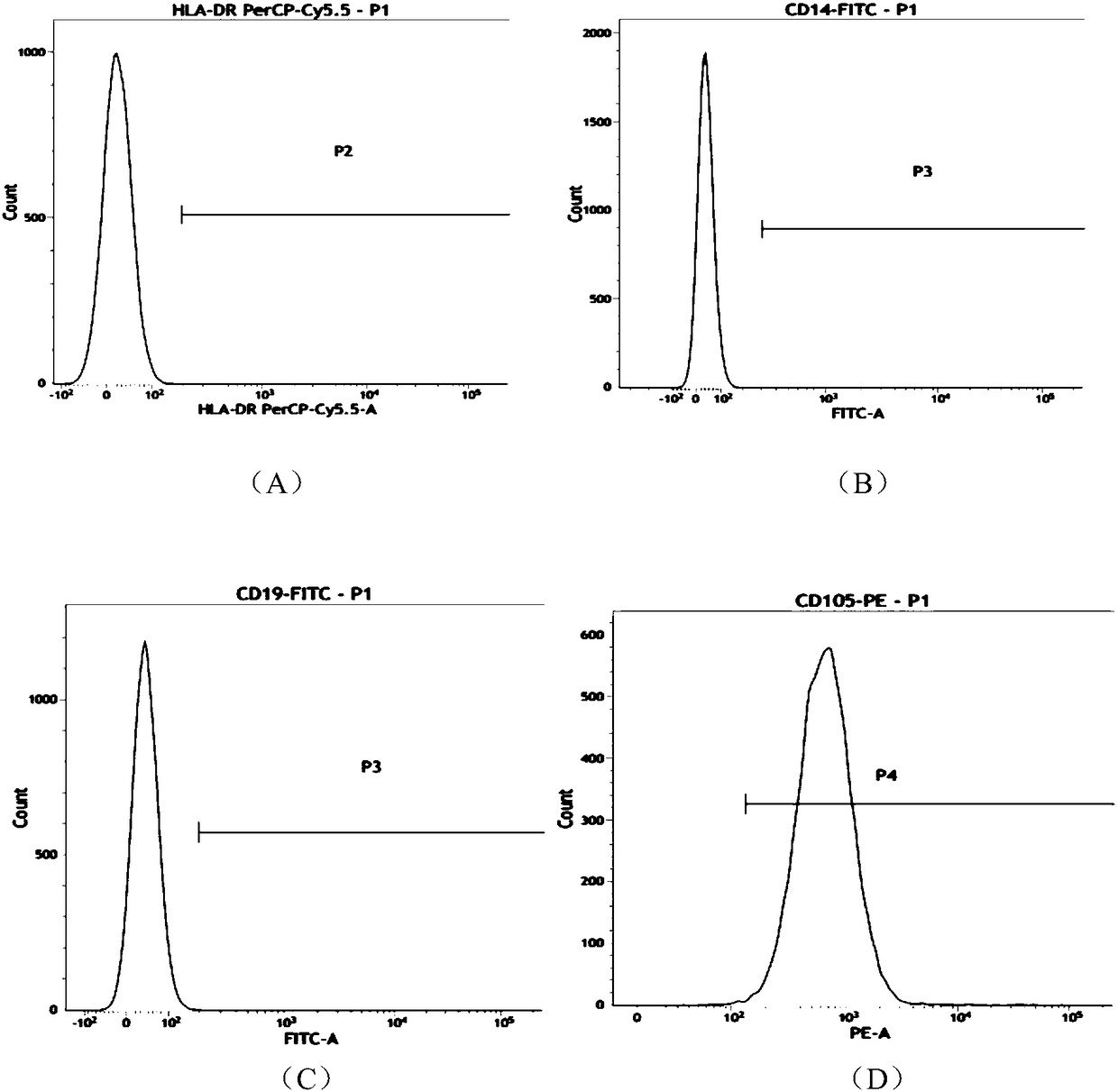
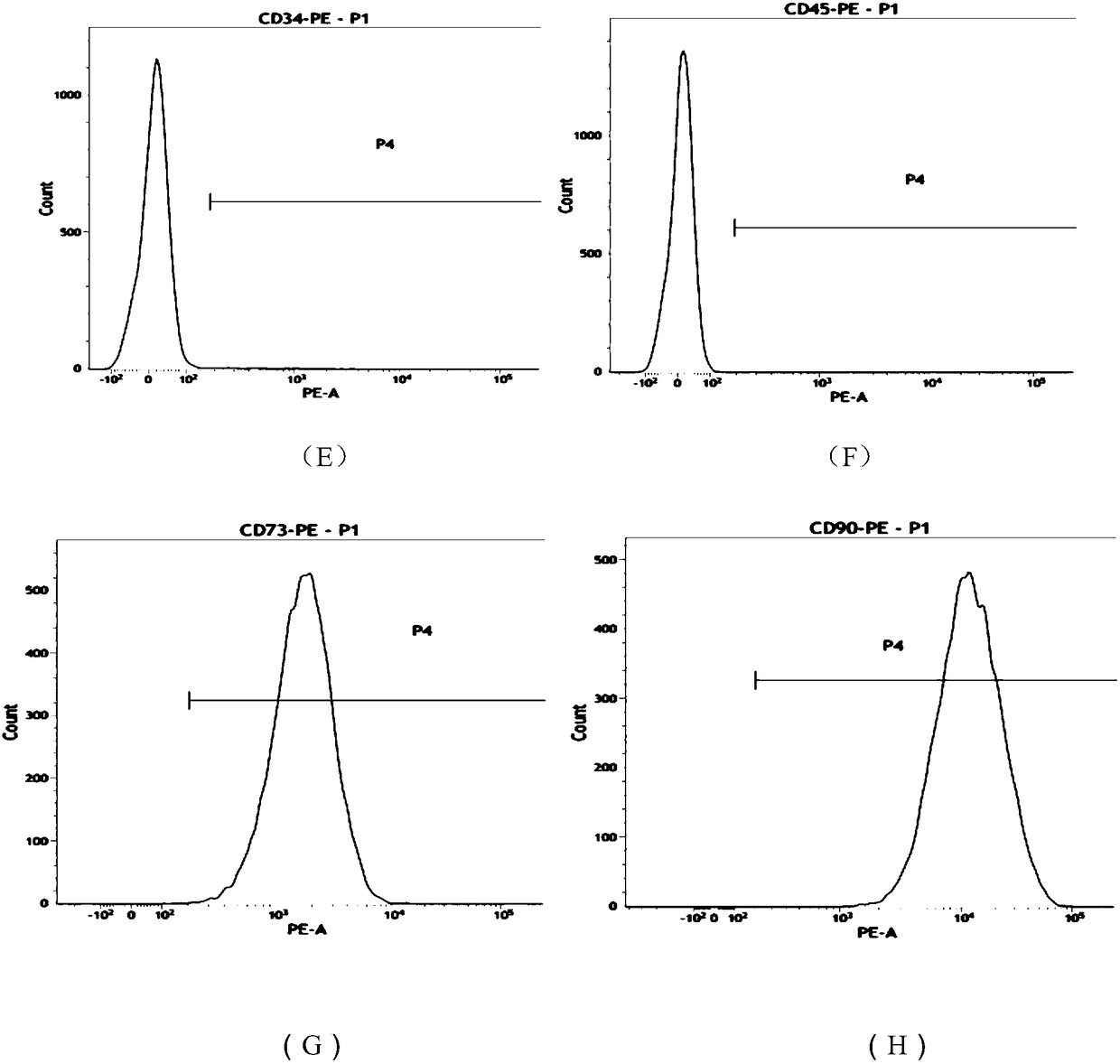
[0053] The morphology of umbilical cord mesenchymal stem cells is like figure 1 As shown, the mesenchymal stem cells with good expansion and proliferation status were taken, and the cells were digested into single cells with TrypLE Express digestion enzyme; then washed with PBS containing 1% BSA to prepare the cells containing 5×10 6 A single cell suspension of umbilical cord mesenchymal stem cells, and labeled CD90, CD73, CD105, CD34, CD45, CD14, CD19, HLA-CR flow cytometry antibody (BD Company, USA), each tube added 5μl at room temperature Incubate in light for 20 minutes; after washing twice with PBS containing 1% BSA, resuspend the cells to 400 μl, and perform flow cytometry. The results are as follows figure 2 As shown, (A) to (H) are HLA-CR, CD14, CD19, CD105, CD34, CD45, CD73, CD90 antibodies, respectively. From Table 1, it can be ...
Embodiment 2
[0061] Example 2 Preparation of the second reagent
[0062] (1) Soak the fresh oxtail in 75% alcohol for 10 minutes, and then repeatedly rinse with sterile normal saline;
[0063] (2) Peel off the tail tendon, remove the muscle membrane and fascia, and place it in a sterile dish;
[0064] (3) Cut the tail tendon, immerse it in 0.05mol / L acetic acid solution and shake at 4℃ for 48 hours;
[0065] (4) Move it into a pulverizer to smash, and then put it in a 4℃ refrigerator for secondary expansion;
[0066] (5) After 24 hours, filter with a 200-mesh screen and collect the filtrate to obtain a collagen solution;
[0067] (6) In a 6-well plate, add 5ml of collagen solution to each well to spread it flat in the well;
[0068] (7) Pre-freeze the well plate in a refrigerator at -30°C for 6 hours, and then vacuum dry for 18-24 hours to obtain a collagen sponge;
[0069] (8) Place the collagen sponge in a 10 cm petri dish, and add 30 mL of 0.25% glutaraldehyde solution to the petri dish for pre-cros...
Embodiment 3
[0075] Example 3 Differentiation of mesenchymal stem cells into epidermal cells in vitro
[0076] 1. Differentiation of mesenchymal stem cells into epidermal cells in vitro
[0077] On a 6-well plate pre-covered with Matrigel (BD Company, USA), the planting cell density is 6×10 4 Cells / cm 2 Umbilical cord mesenchymal stem cells, add 2ml low-sugar DMEM medium containing 10% fetal bovine serum to each well at 37℃, 5% CO 2 Cultivate in an incubator until the cells reach 50% fusion, switch to 2ml epidermal induction conditioned medium for induction for 7 days, and change half of the medium every other day.
[0078] Epidermal induction conditioned medium formula: (DMEM:DF12=1:1 (purchased from Sigma), 20ng / ml epidermal growth factor (purchased from R&D), 15ng / ml basic fibroblast growth factor (purchased from R&D), 1% insulin-transferrin-selenium complex (purchased from Sigma), 0.1 μM dexamethasone (purchased from Sigma), 100 U / ml penicillin and 100 μg / ml streptomycin.
[0079] 2. Morpholog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com