Fimasartan compound
A technology for fimasartan and compounds, applied in the field of fimasartan compounds and their crystal forms, can solve the problems of difficult separation of impurities, low yield, unsatisfactory overall yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
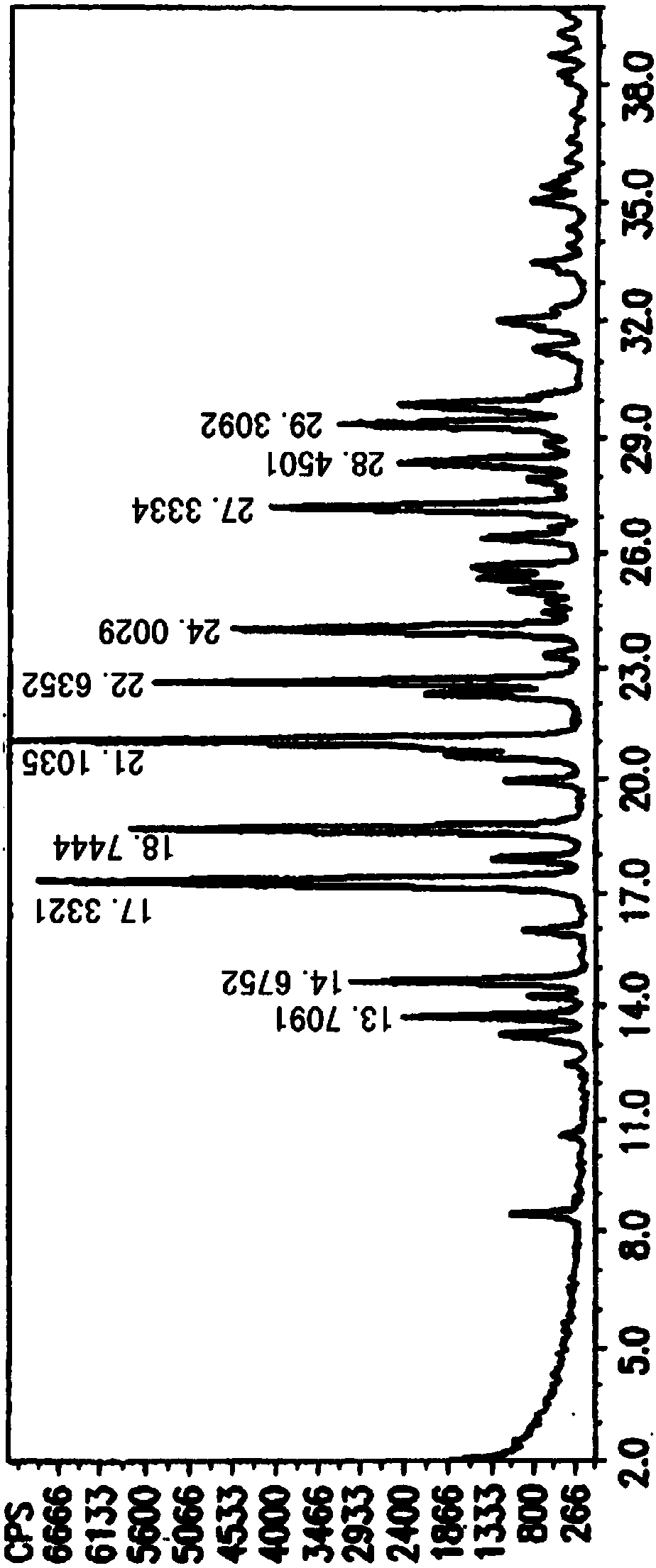
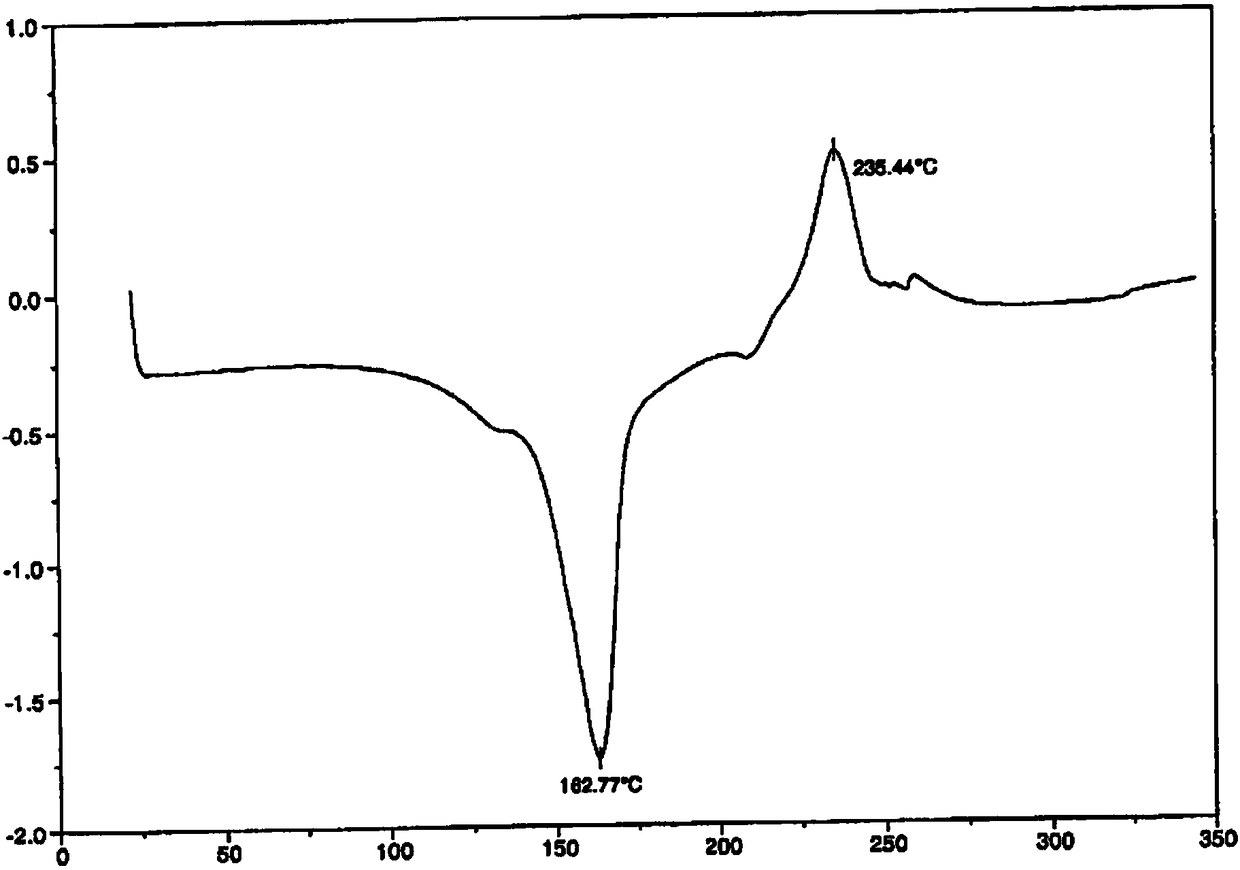
[0018] In the reaction flask, add Fimasartan potassium (2.95kg, prepared according to Lu Xiusheng's "Synthesis of Fimasartan" "Chemical Reagent", 2016, 38 (3), 283-286), 80% ethanol (4.5L ), after the addition, stir and heat up to 75°C-80°C. After the solution is clear, naturally cool down to room temperature and stir overnight. The next day, the mother liquor was cooled to -5°C-0°C and stirred for 2 hours, filtered with suction, the filter cake was washed with purified water (0.2L×2), filtered with suction, and the filter cake was air-dried at 30-35°C for 3 hours to obtain Fimasartan Potassium white solid 2.60Kg, HPLC purity 99.14%. Powder X-ray diffraction: using Cu-K radiation, the X-ray powder diffraction pattern of this compound is shown in the attached figure 1 . Differential scanning calorimetry analysis: The differential thermal analysis spectrum of this compound is shown in the attached figure 2 .
Embodiment 2
[0020] In the reaction flask, add Fimasartan potassium raw material (2.85kg is prepared according to Lu Xiusheng's "Synthesis of Fimasartan" "Chemical Reagent", 2016, 38 (3), 283-286), 63% acetone (3.8L ), after the addition, stir and heat up to 65°C-70°C. After the solution is clear, naturally cool down to room temperature and stir overnight. The next day, the mother liquor was cooled to -5°C-0°C and stirred for 2.5 hours, filtered with suction, the filter cake was washed with purified water (0.2L×2), filtered with suction, and the filter cake was air-dried at 30-35°C for 3 hours to obtain Fimarsa Potassium Tan is 2.31Kg white solid, and its HPLC purity is 99.25%. . Powder X-ray diffraction: using Cu-K radiation, the X-ray powder diffraction pattern of this compound is shown in the attached figure 1 . Differential scanning calorimetry analysis: The differential thermal analysis spectrum of this compound is shown in the attached figure 2 .
[0021] Table 1 embodiment ful...
Embodiment 3
[0024] prescription:
[0025]
[0026] Preparation Process:
[0027] (1) Fimasartan is mixed with croscarmellose sodium, sieved, then mixed with microcrystalline cellulose and silicon dioxide, added magnesium stearate, and mixed uniformly;
[0028] (2) direct compression to get the fimasartan tablet of embodiment 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com