High-throughput kit for simultaneously detecting three avian virus diseases, and detecting method and application thereof
A kit, the technology of influenza virus, applied in the field of molecular biology detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] [Example 1] Design and screening of primers and probes
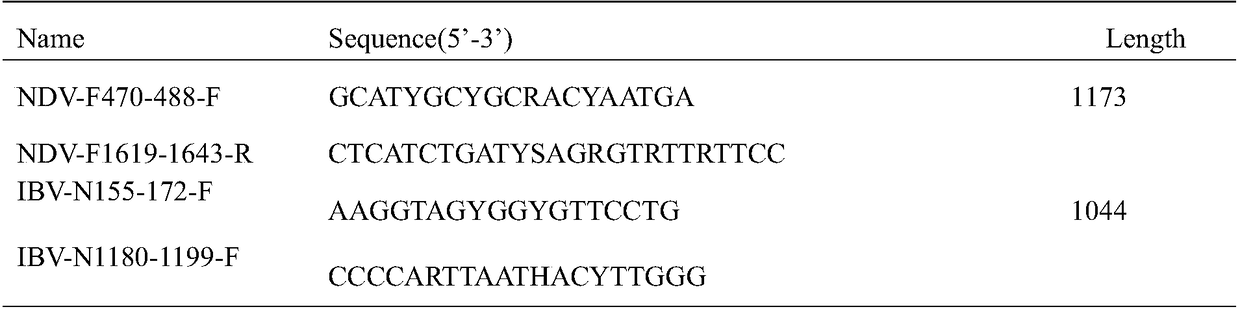
[0068] According to the M gene of AIV in GenBank, the hemagglutinin gene sequence of H5, H7 and H9 subtype avian influenza virus, the nucleocapsid protein gene (N gene) of IBV and the fusion protein gene (F gene) of NDV, carry out with DNAMAN software For homology analysis, use Primer Express5.0 software to design specific primers and gene probes in its conserved regions. For each probe, design 2 or more primers and probes for screening. For primers and probes with good cross-reaction and amplification efficiency, the 5' upstream of the primers are all modified with Biotin. The primers and probes of each subtype are shown in Table 2 below.
Embodiment 2
[0069] [Example 2] Preparation of positive standard
[0070] A. Extract the total RNA of IBV and NDV virus samples respectively;
[0071] B, the IBV and NDV obtained in the step A are reverse-transcribed respectively to obtain the cDNA of IBV and NDV virus;
[0072] C. Use the cDNA obtained in step B as a template, and use the nucleotide sequences shown in Table 1 below as upstream primers and downstream primers to amplify IBV and NDV virus-specific target fragments to obtain IBV-N and NDV-F Specific target fragments;
[0073] D. Connect the IBV and NDV-specific target fragments obtained in step C to the pMD18T vector and transfer them into Escherichia coli. The plasmid laboratory that connects the fragments of H5, H7, H9 and M to the pMD18T vector already exists, and the preparation process As above, the primer sequences are shown in Table 1 below, and equal volumes of the appealing plasmids were mixed to obtain positive standards with a final concentration of 0.1 mg / ml of ...
Embodiment 3
[0074] [Example 3] The kit that can simultaneously detect NDV, IBV and AIV and H5, H7, H9 subtype influenza virus thereof
[0075] Contains the following components:
[0076] (1) 48-hole gene reaction plate prepared with probes, five white films;
[0077] (2) Lotion 1 (20×SSC), volume 100mL;
[0078] (3) The volume of lotion 2 (10% SDS, 10g of SDS dissolved in water to 100ml) is 30mL;
[0079] (4) The volume of lotion 3 (1M sodium citrate) is 100mL;
[0080] (5) 5 μl of forward primer (4nmol / μl), 5 μl of reverse primer (4nmol / μl);
[0081] (6) POD (Streptavidin-Horseradish Peroxidase): 50μL;
[0082] (7) One-step multiplex RT-PCR reagents, including 2 × One Step Q Probe Mix3mL, OneStep Q Probe Enzyme Mix300μl and RNase-free water3mL in the reagent; the above-mentioned reagents are commercially available one-step multiplex RT-PCR kits (HiScript Reagents in II One Step qRT-PCR Probe Kit product number: Q222-01).
[0083] (8) Positive control sample: 200 μL of the positive ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com