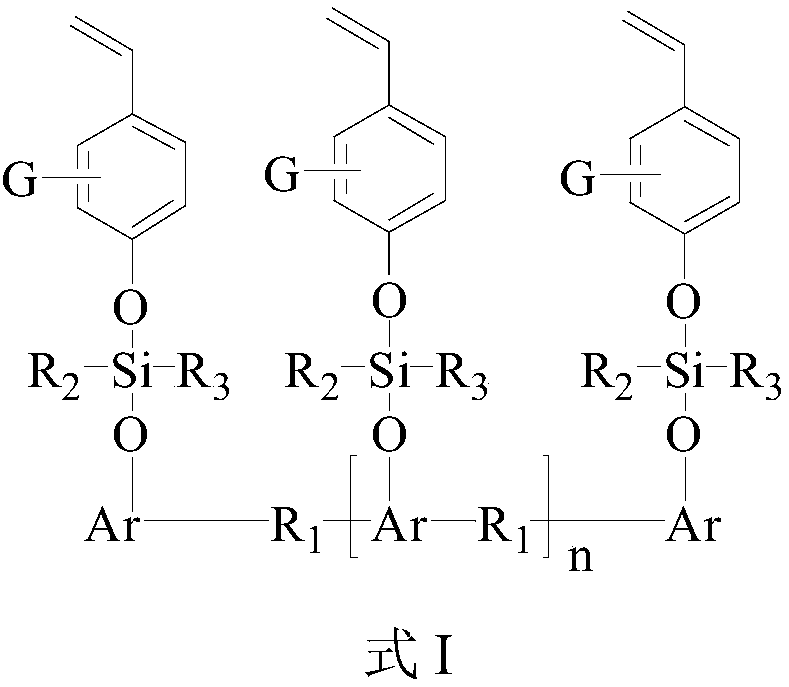
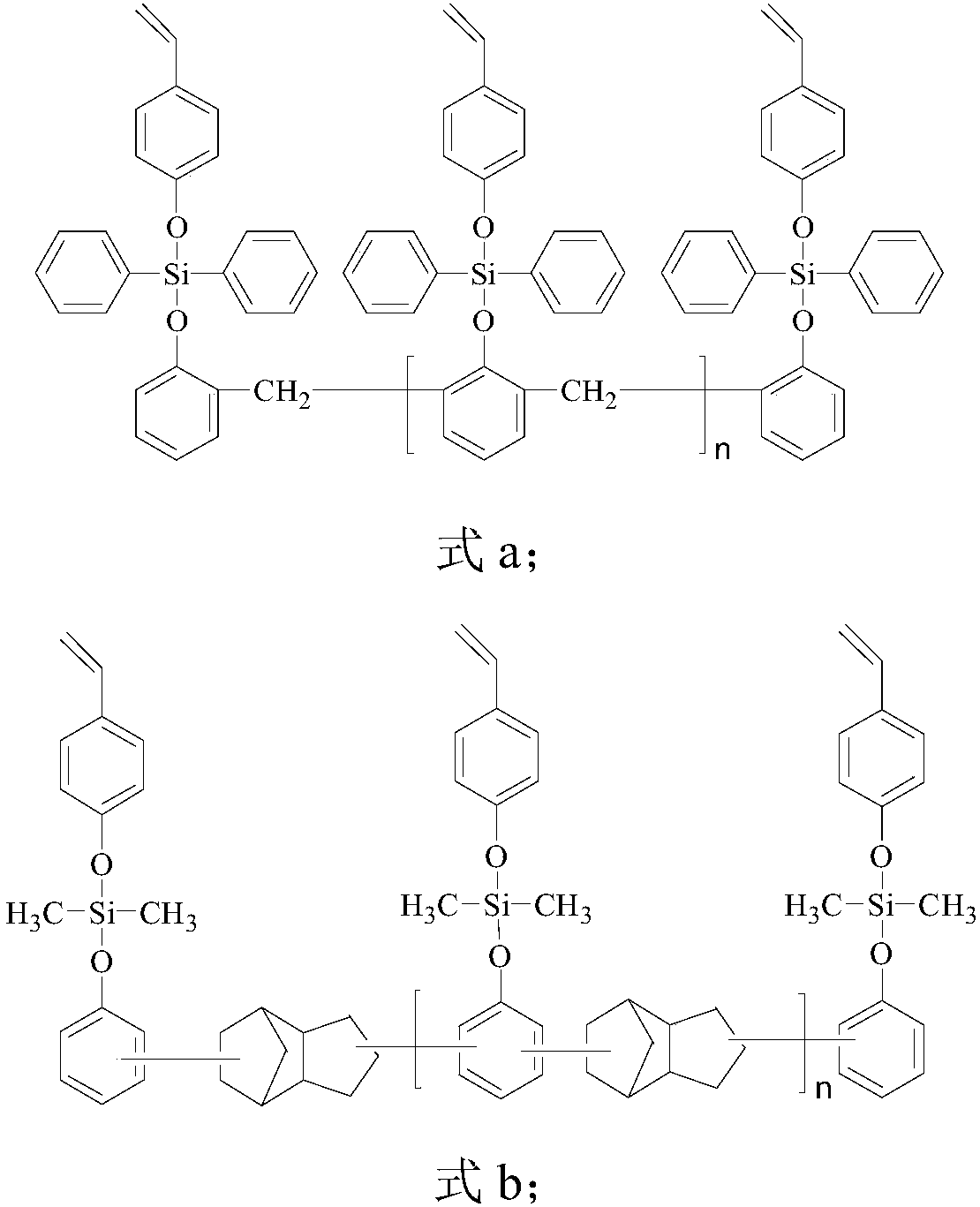
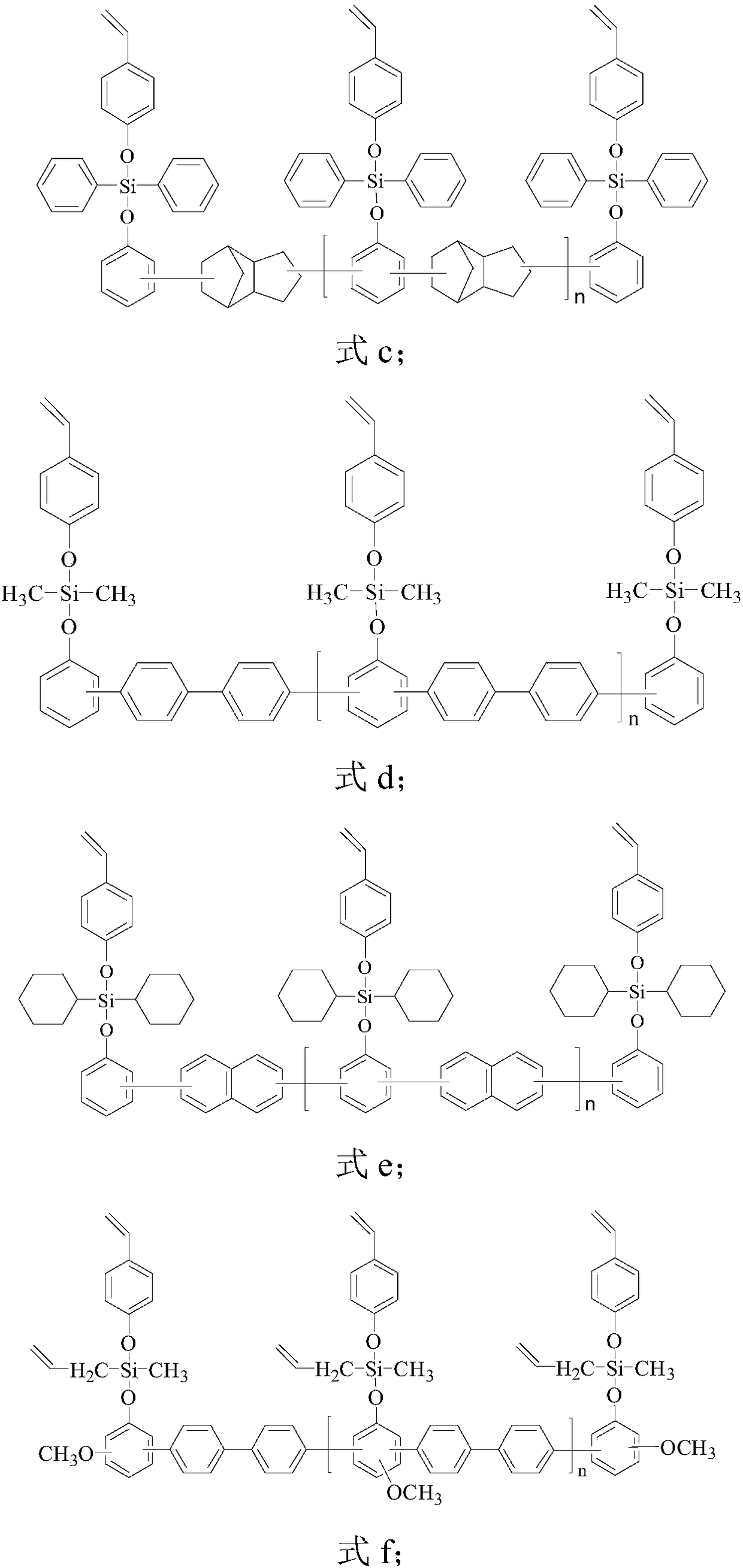
Styryl siloxy phenolic resin, and preparation method and application thereof
A technology based on siloxy phenolic resin and phenolic resin, which is applied in the field of styrene-based siloxy phenolic resin and its preparation, and can solve the problems of undisclosed performance improvement of polyphenylene ether, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Stir 21 parts by weight of phenol-formaldehyde phenolic resin and 1000 mL of anhydrous tetrahydrofuran in a reaction kettle equipped with a stirrer, dropping funnel, thermometer and air duct (nitrogen) until completely dissolved into a uniform solution, and continue nitrogen for 0.5- The water vapor in the reaction kettle was removed for 1 hour, and nitrogen gas was kept flowing throughout the reaction process. Keep the temperature in the reactor below 20° C., and then slowly add 53 parts by weight of diphenyldichlorosilane dropwise. After the dropwise addition, the reaction kettle was kept below 20°C for 8 hours, and then the temperature was raised to 55°C for 3 hours. Then, 26 parts by weight of p-hydroxystyrene was added dropwise into the reactor, and reacted at 55° C. for 5 hours. After the reaction finishes, THF is removed by distillation under reduced pressure to obtain a novolac resin (modified resin a) modified by styrylsiloxy groups. The styryl equivalent of t...
Embodiment 2
[0099] 42 parts by weight of dicyclopentadiene-type phenolic resin and 1000mL of anhydrous tetrahydrofuran were stirred in a reactor equipped with a stirrer, dropping funnel, thermometer and gas guide tube (nitrogen) until completely dissolved into a uniform solution, and nitrogen was continued. The water vapor in the reactor was removed in 0.5-1 hour, and nitrogen was kept flowing during the whole reaction process. At the same time, the temperature in the reactor was kept below 20° C., and then 30 parts by weight of dimethyldichlorosilane was slowly added dropwise. After the dropwise addition, the reaction kettle was kept below 20°C for 7 hours, and then the temperature was raised to 60°C for 2.5 hours. Then, 28 parts by weight of p-hydroxystyrene was added dropwise into the reaction kettle, and reacted at 58° C. for 6 hours. After reaction finishes, remove tetrahydrofuran by underpressure distillation, obtain the dicyclopentadiene type phenolic resin (modified resin b) of s...
Embodiment 3
[0102] 33 parts by weight of dicyclopentadiene-type phenolic resin and 1000mL of anhydrous tetrahydrofuran were stirred in a reactor equipped with a stirrer, dropping funnel, thermometer and air duct (nitrogen) until completely dissolved into a uniform solution, and the nitrogen was continued. The water vapor in the reactor was removed in 0.5-1 hour, and nitrogen was kept flowing during the whole reaction process. At the same time, the temperature in the reactor was kept below 20° C., and then 46 parts by weight of diphenyldichlorosilane was slowly added dropwise. After the dropwise addition, the reaction kettle was kept below 20°C for 8 hours, and then the temperature was raised to 55°C for 3 hours. Then, 28 parts by weight of p-hydroxystyrene were added dropwise into the reaction kettle, and reacted at 55° C. for 5 hours. After reaction finishes, remove tetrahydrofuran by underpressure distillation, obtain the dicyclopentadiene type phenolic resin (modified resin c) of styr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com