A kind of method of synthesizing α-amino boron compound
A boron compound and amino technology, which is applied in the field of synthesizing α-amino boron compounds, can solve problems such as poor functional group compatibility, and achieve the effects of strong functional group compatibility, safe reaction operation, and good atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
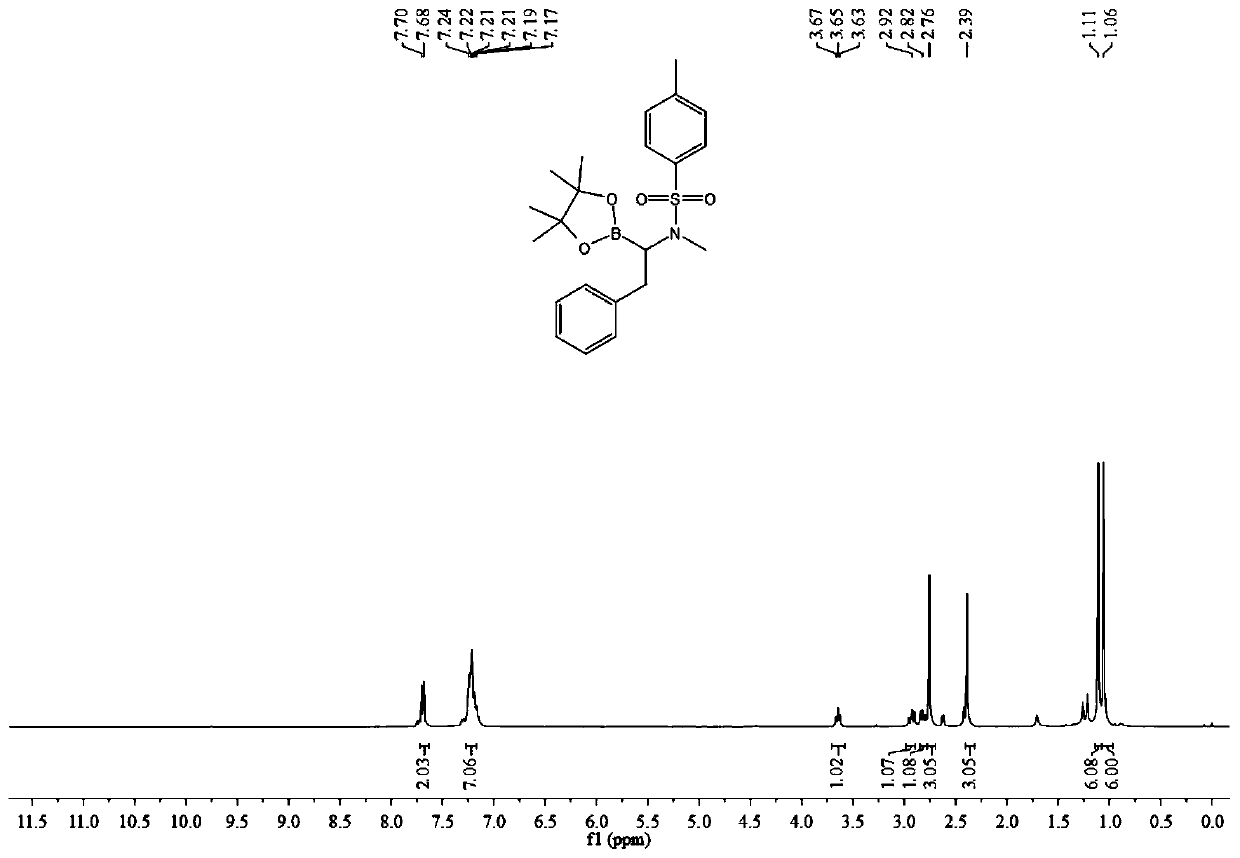
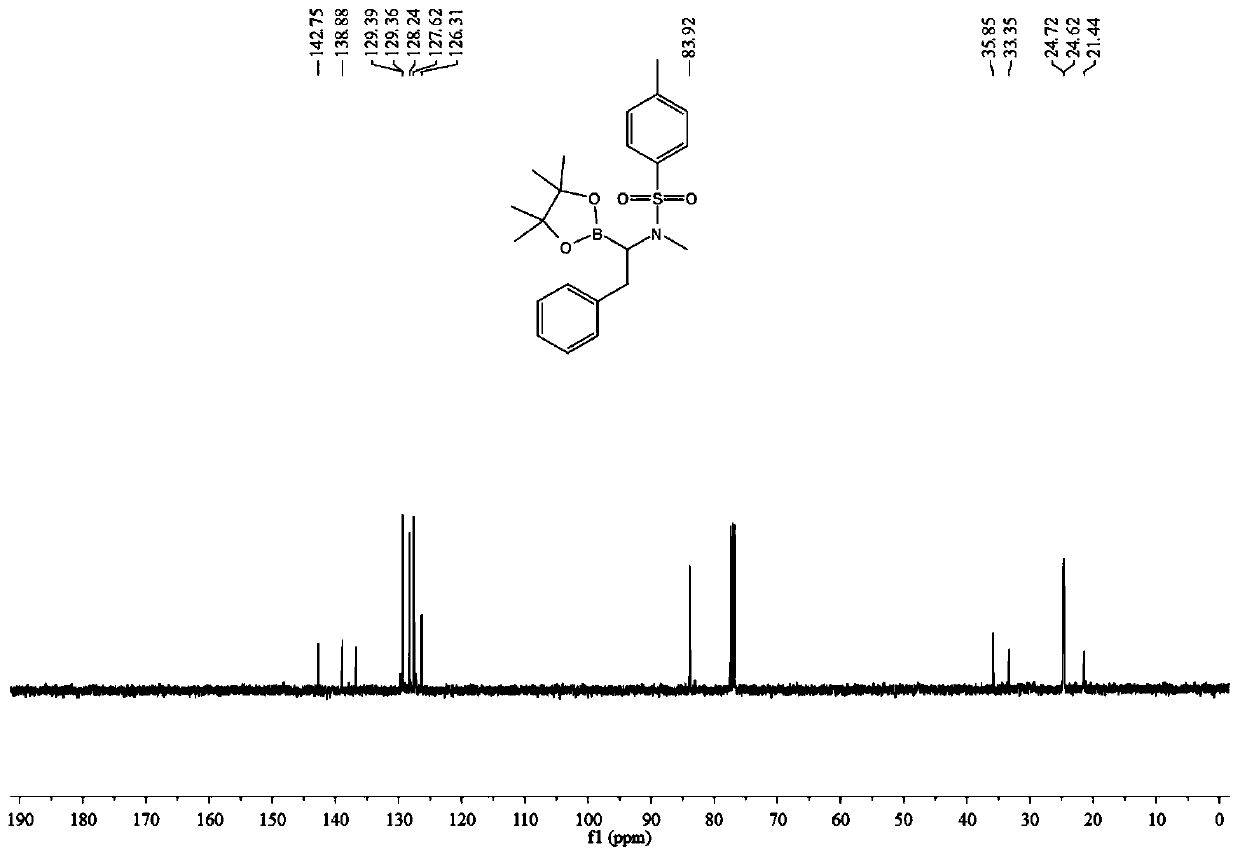
[0034] (1) Add 0.3 mmol of N-methyl-N-(phenylethynyl) p-toluenesulfonamide, 0.36 mmol of pinacol diborate, 0.03 mmol of cuprous chloride and 0.036 mmol of Mole of 4,5-bisdiphenylphosphine-9,9-dimethylxanthene, 0.045 mmol of sodium tert-butoxide, add 2 mL of toluene as a solvent, stir and react at room temperature for 12 hours, then filter, The solvent was spin-dried to obtain a crude product.
[0035] (2) After adding 3 mL of ethyl acetate to the crude product of step (1), transfer it to the autoclave, add 0.03 mmol of palladium / carbon, and then feed hydrogen at 20 atmospheres, and stir at a temperature of 60 degrees for 12 After 1 hour, filter, spin dry, add 2mL of petroleum ether to dissolve, take the petroleum ether layer, repeat three times, combine the petroleum ether layer, remove the solvent by rotary evaporation under reduced pressure, and then passivate through the previously prepared silica gel and water mass ratio of 1:0.35 The target product was obtained by separa...
Embodiment 2
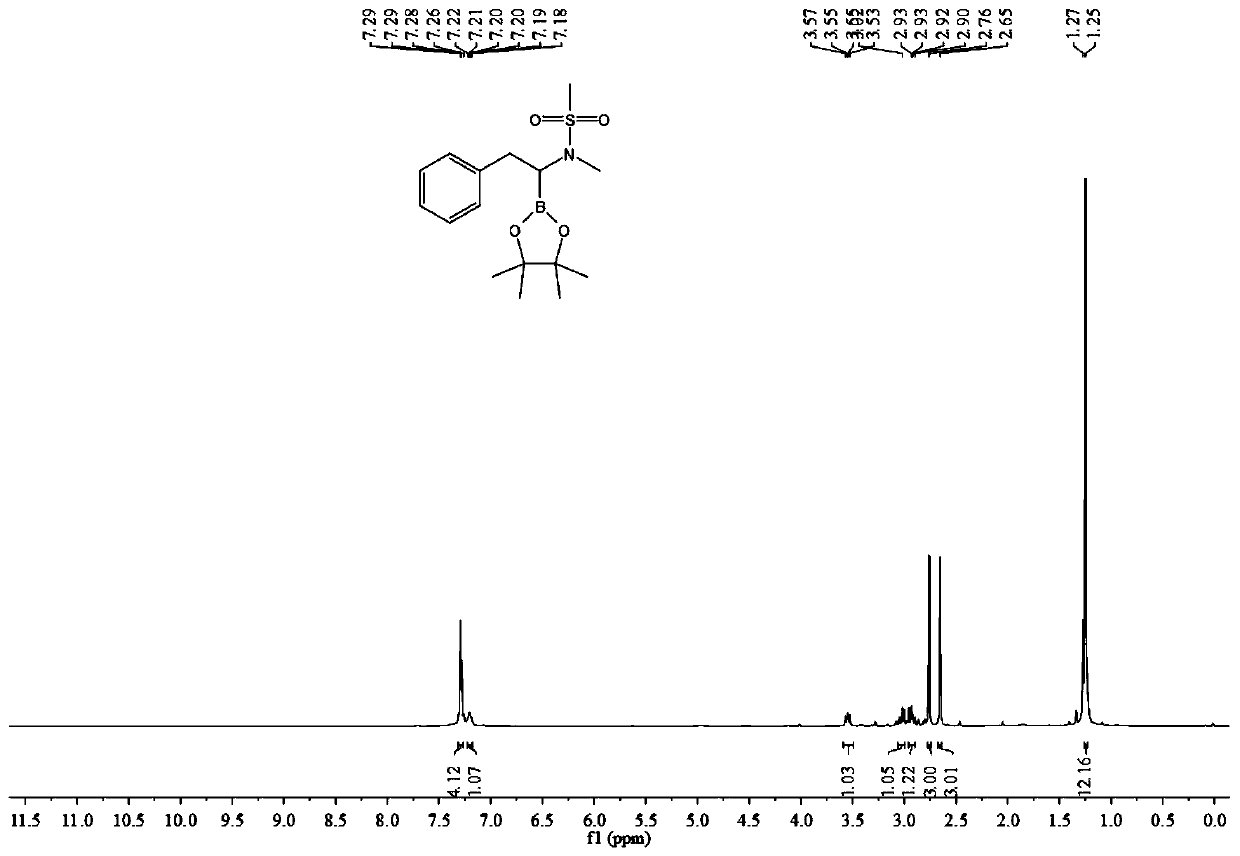
[0044] (1) In a 25mL test tube, add 0.3 mmol of N-methyl-N-(phenylethynyl) methanesulfonamide, 0.36 mmol of pinacol biborate, 0.03 mmol of cuprous chloride and 0.036 mmol of 4,5-bisdiphenylphosphine-9,9-dimethylxanthene, 0.045 mmol of sodium tert-butoxide, adding 2 mL of toluene as a solvent, stirring and reacting at room temperature for 12 hours, filtering, spinning The solvent was dried to obtain the crude product.
[0045] (2) After adding 3 mL of ethyl acetate to the crude product of step (1), transfer it to the autoclave, add 0.03 mmol of palladium / carbon, and then feed hydrogen at 20 atmospheres, and stir at a temperature of 60 degrees for 12 After 1 hour, filter, spin dry, add 2mL of petroleum ether to dissolve, take the petroleum ether layer, repeat three times, combine the petroleum ether layer, remove the solvent by rotary evaporation under reduced pressure, and then passivate through the previously prepared silica gel and water mass ratio of 1:0.35 Silica gel colum...
Embodiment 3
[0054] (1) Add 0.3 mmol of 3-(phenylethynyl) oxazolidin-2-one, 0.36 mmol of pinacol biborate, 0.03 mmol of cuprous chloride and 0.036 mmol of 4,5-bisdiphenylphosphine-9,9-dimethylxanthene, 0.045 mmol of sodium tert-butoxide, add 2 mL of toluene as a solvent, stir and react at room temperature for 12 hours, filter and spin dry solvent, the crude product was obtained.
[0055] (2) After adding 3 mL of ethyl acetate to the crude product of step (1), transfer it to the autoclave, add 0.03 mmol of palladium / carbon, and then feed hydrogen at 20 atmospheres, and stir at a temperature of 60 degrees for 12 After 1 hour, filter, spin dry, add 2mL of petroleum ether to dissolve, take the petroleum ether layer, repeat three times, combine the petroleum ether layer, remove the solvent by rotary evaporation under reduced pressure, and then passivate through the previously prepared silica gel and water mass ratio of 1:0.35 Silica gel column separation and purification to obtain the target p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com