Preparation and application of alpha-polyglutamic acid-cisplatin compound
A technology of polyglutamic acid and complexes, which is applied in the field of preparation of anticancer drugs, can solve the problems of neurotoxicity and gastrointestinal adverse reactions, high liver and kidney toxicity, poor selectivity, etc., and achieve good antitumor effect and drug loading The effect of high dosage and high utilization rate of cisplatin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
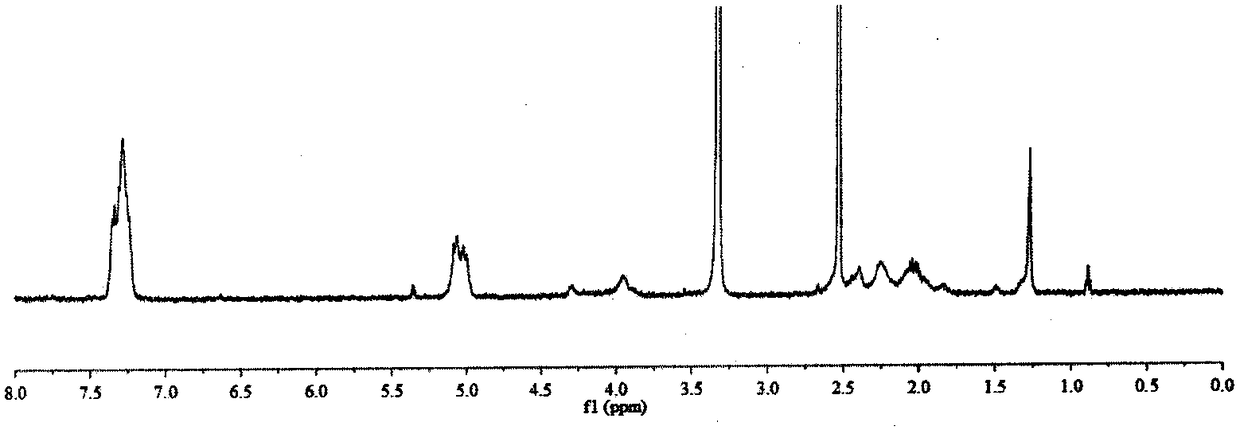
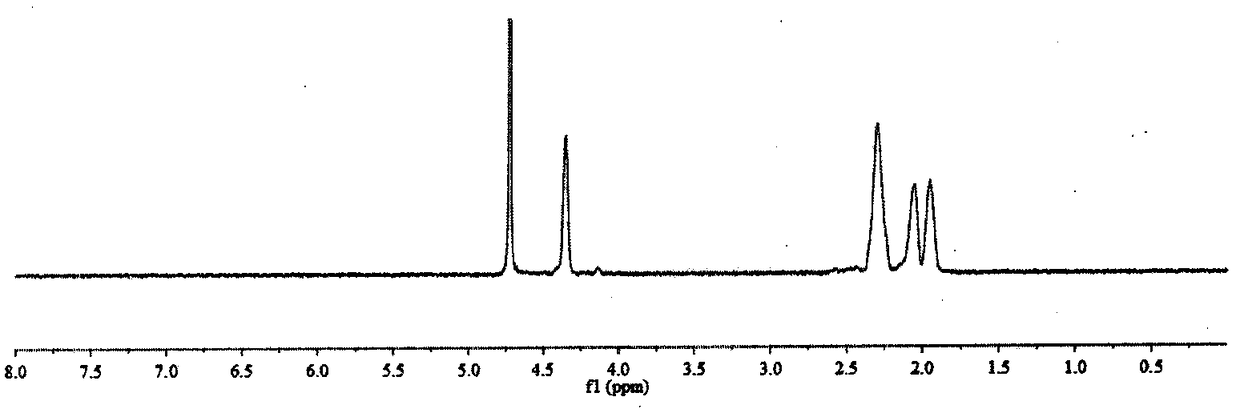
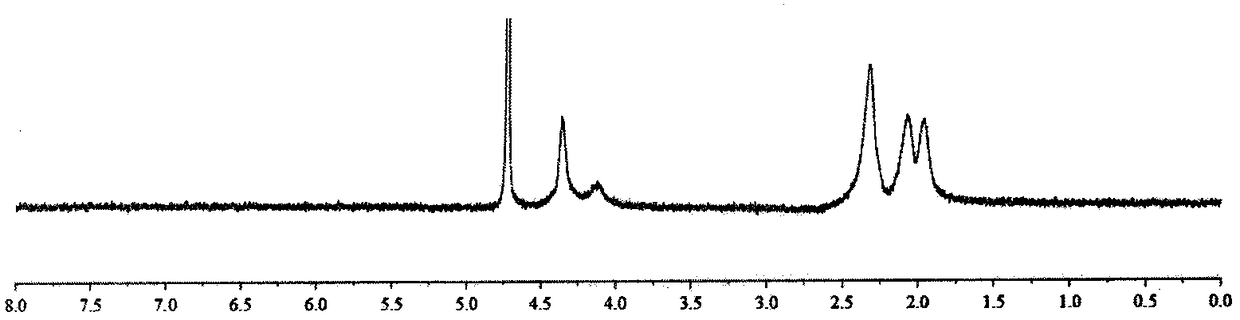
[0033] Preparation method and characterization of α-polyglutamic acid-cisplatin complex
[0034] Preparation method: Dissolve γ-benzyl-L-glutamic acid-N-carboxylic acid anhydride in dioxane, add dropwise triethylamine, N 2 After reacting at room temperature for four days, the reaction solution was added dropwise to an appropriate amount of ice ethanol, the precipitate was centrifuged and washed with ethanol three times, and finally the precipitate was vacuum dried at 45° C. to obtain white block polybenzyl glutamate. The polybenzyl glutamate was dissolved in dichloroacetic acid, and an appropriate amount of 33% HBr / acetic acid solution was added to react at room temperature for 4 hours. The reaction solution was dropped into ice ether, the precipitate was centrifuged, and washed with ether three times. The precipitate was dried in vacuum, then dissolved with an appropriate amount of saturated sodium bicarbonate, and lyophilized after dialysis to obtain α-polyglutamic acid. Disso...
Embodiment 2
[0039] Determination of cisplatin in α-polyglutamic acid-cisplatin complex
[0040] A graphite furnace atomic absorption spectrometer was used to determine the content of cisplatin in the composite. Detection conditions: wavelength 265.9nm, lamp current 6mA; slit width 0.2mm; sample volume 20μl; protective gas is high purity argon (Ar, 0.2L / min). Take the concentration as 10 6 The ng / ml platinum standard solution is the mother solution. The mother solution is diluted into a standard solution with a concentration of 40, 80, 120, 160, 200 μg / ml. The concentration is the abscissa and the absorption value is the ordinate to calculate the standard curve. The absorption value of the complex aqueous solution was determined after digestion and dilution with nitric acid. The content of cisplatin can be calculated from Biaoqu. It was determined that the utilization rate of cisplatin in Example 1 was greater than 80%, and the drug loading was greater than 28%. The calculation formula for ...
Embodiment 3
[0044] Study on the release of α-polyglutamic acid-cisplatin compound in vitro
[0045] Put 5ml of α-polyglutamic acid-cisplatin complex solution (0.2mg / ml) in a dialysis bag (with a molecular weight cut-off of 3.5kDa), and add 25ml of a PBS solution with a chloride ion concentration of 150mmol / L (pH 7.4). ) Mix and place in a constant temperature shaking box at 37°C. Take 1ml samples at different time periods (1, 2, 4, 6, 8, 12, 24, 36, 48, 72, 96, 120, 144h) and save them for later use. At the same time, add 1ml of new PBS solution and use graphite furnace atom The absorption meter detects the amount of free cisplatin released and calculates its cumulative release rate. The results are shown in Figure 4 .
[0046] Figure 4 The results show that the α-polyglutamic acid-cisplatin complex has no burst release under physiological pH conditions and can be released slowly, and the cumulative release rate of 144h reaches 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com