Albumin-binding antineoplastic drug-maleimide molecular prodrug
A technology of albumin-binding and maleimide, which is applied in the direction of antineoplastic drugs, drug combinations, and pharmaceutical formulations, can solve problems such as adverse reactions, low solubility of antineoplastic drugs, and lack of tumor targeting, and achieve Prolonged residence time, increased stability, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
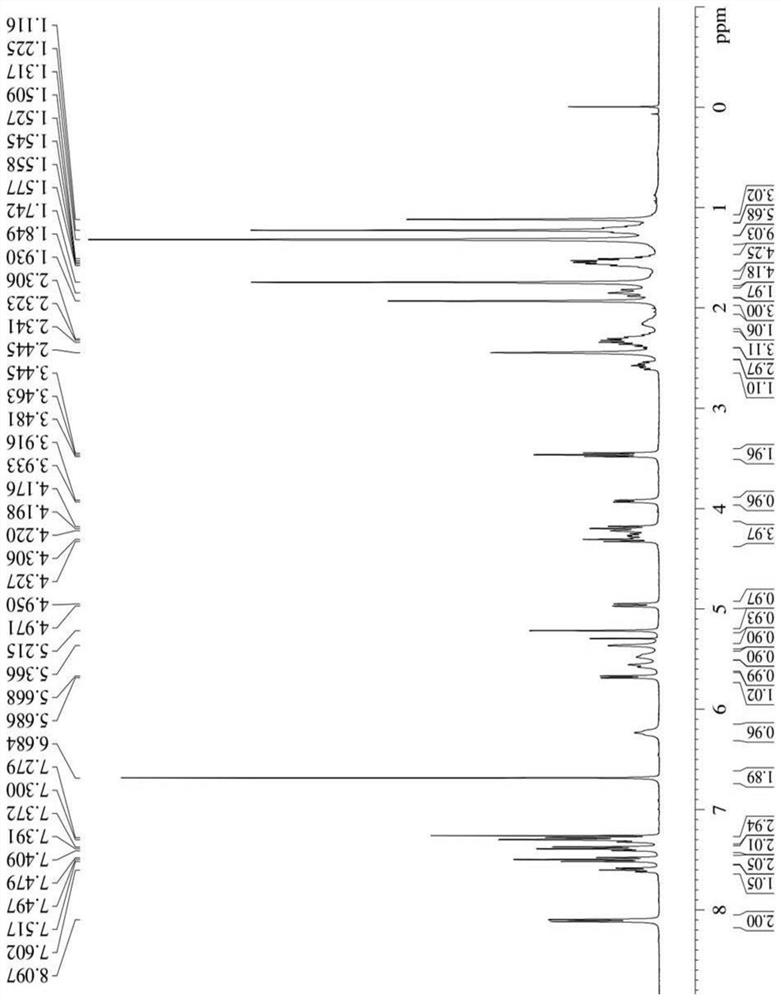
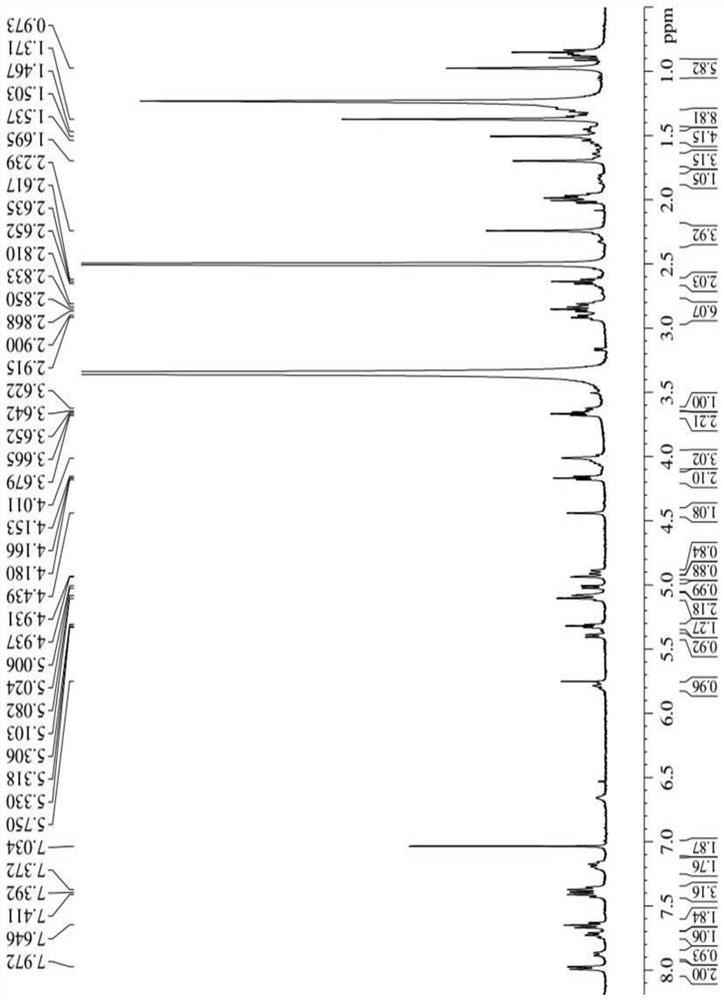
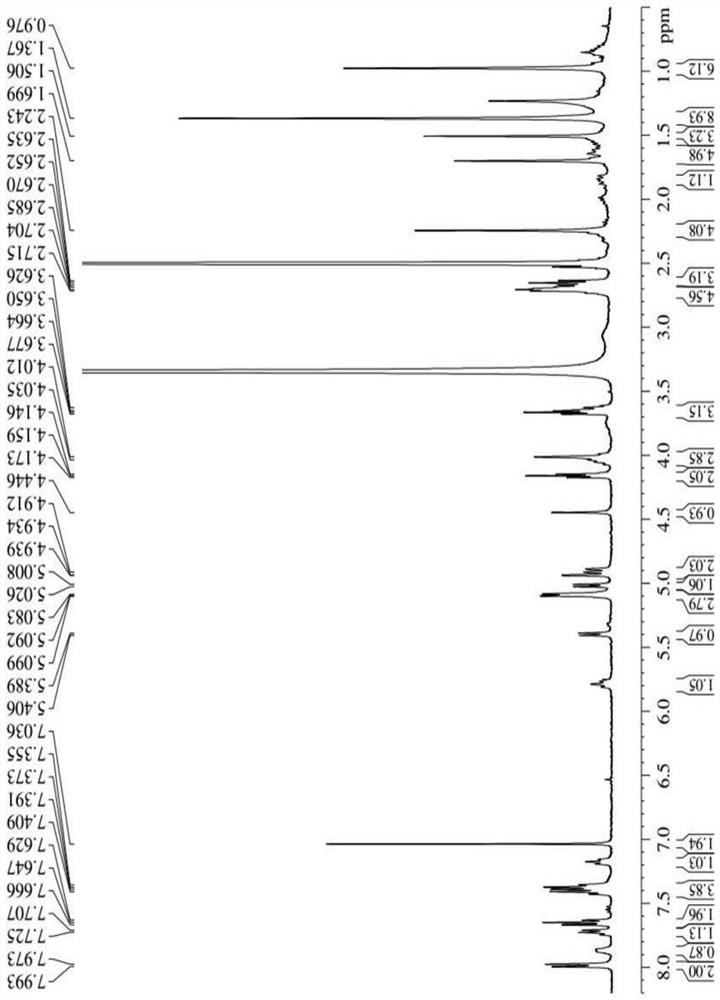
[0041] Synthesis of three different linker-linked docetaxel-maleimide prodrugs.
[0042](a) Synthesis of prodrugs linked by non-sensitive bond ester bonds (abbreviated as DM): get 405mg docetaxel (DTX), dissolve 116mg maleimide caproic acid (EMC) in dichloromethane, add 210mg Dicyclohexylcarboimide (DCC), 12mg 4-dimethylaminopyridine (DMAP), N 2 The reaction was carried out at room temperature under protection for 12 h, and the white powder compound DM was obtained by separation and purification by column chromatography.
[0043] (b) Synthesis of reduction-sensitive disulfide bond-linked prodrug (abbreviated as DSSM): firstly, the key intermediate N-(2-hydroxyethyl)maleimide (compound 4) was synthesized according to the following synthetic route. Get 30g maleic anhydride and dissolve in toluene, add furan, stir at room temperature 24h stop reaction, separate out white solid in the solution, filter and collect, wash three times with toluene, dry, obtain the maleic anhydride (c...
Embodiment 2
[0056] In Vitro Binding Experiments of Three Docetaxel-Maleimide Prodrugs to Bovine Serum Albumin and Plasma
[0057] Appropriate amounts of DM, DSSM and DSM were weighed and added to bovine serum albumin pH 7.4 phosphate buffer so that the concentrations of the prodrug and bovine serum albumin were 300 μM and 700 μM, respectively. Then the mixed solution was incubated in a constant temperature shaker at 37°C, and samples were taken into high-performance liquid phase at specific time points to investigate the binding situation.
[0058] In addition, the bovine serum albumin solution was replaced with rat plasma, and the above operation was repeated to investigate the binding of the three prodrugs to plasma albumin in vitro. And use excess maleimide caproic acid to carry out competitive binding experiments: first add excess maleimide caproic acid to the plasma and incubate for 1 hour, then add each prodrug, take a point into the liquid phase, and investigate the The binding st...
Embodiment 3
[0061] In vitro release experiments of three albumin-prodrug complexes under different redox conditions
[0062] Using the conditions of the above-mentioned binding experiment, the three prodrugs were incubated with bovine serum albumin solution for 2 hours, and no free prodrug was found in the sample injection, that is, the binding was complete. The solution was then lyophilized to obtain three albumin-prodrug complexes (BSA-DM, BSA-DSSM and BSA-DSM). Then use pH 7.4 phosphate buffer containing 30% ethanol as the release medium to investigate the in vitro release of three albumin-prodrug complexes: 500 mg) was added to 1 mL of pH 7.4 phosphate buffer solution, transferred to a dialysis bag, placed in 30 mL of release medium, and sampled at the set time points at 37°C, and released by high performance liquid chromatography concentration of docetaxel. A certain concentration of hydrogen peroxide (H 2 o 2 ) or dithiothreitol (DTT), respectively, to investigate the release of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com