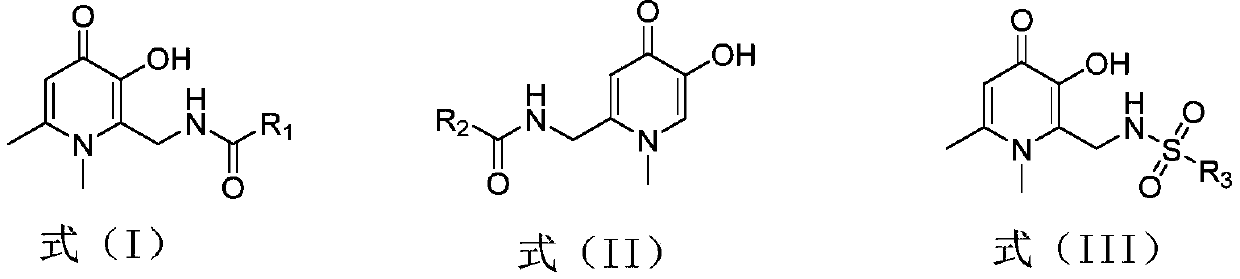
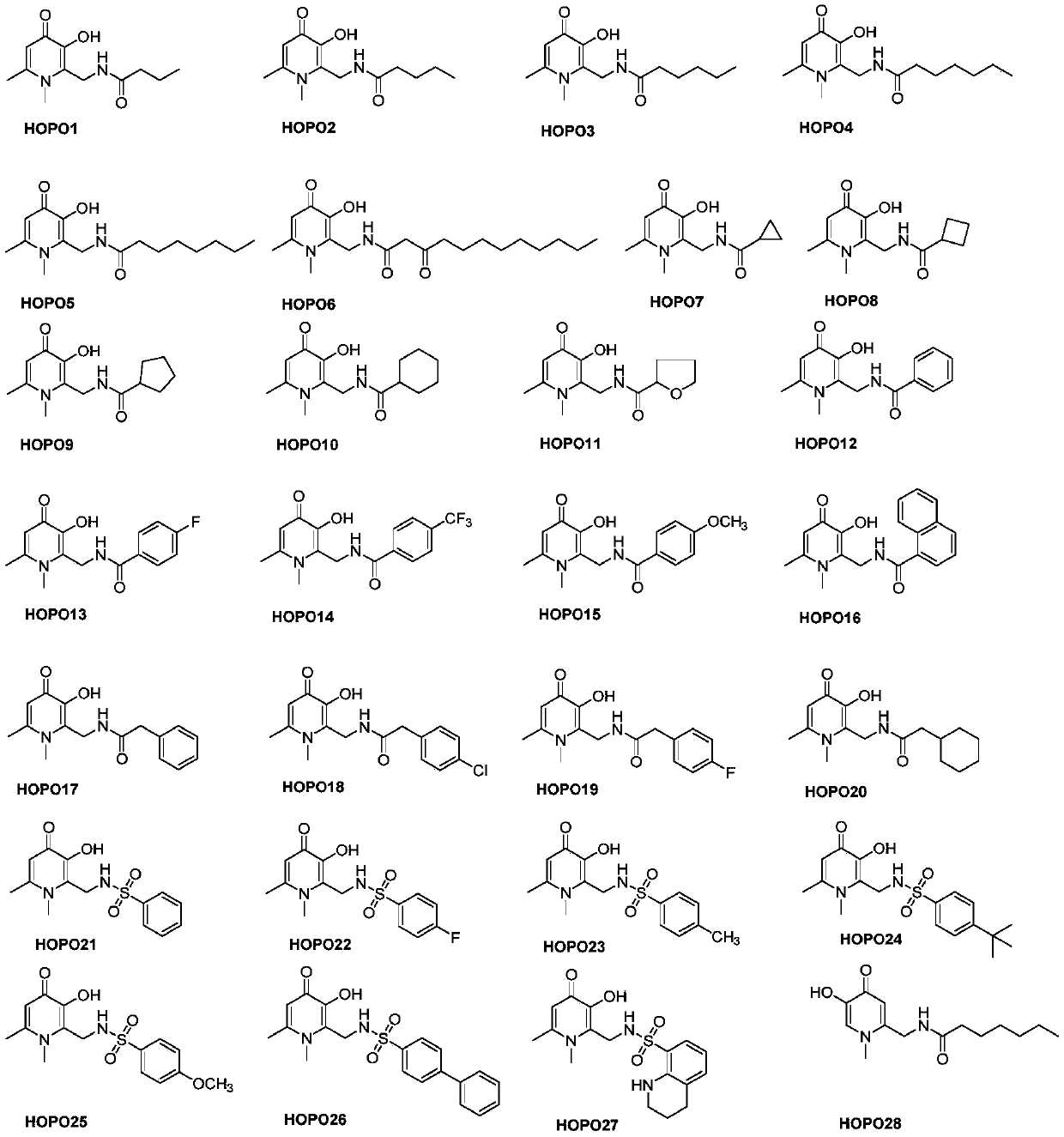
A kind of hydroxypyridone compound and its preparation method and application
A technology of hydroxypyridones and compounds, applied in the field of medicine, can solve problems such as ineffectiveness and reduced efficacy of antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
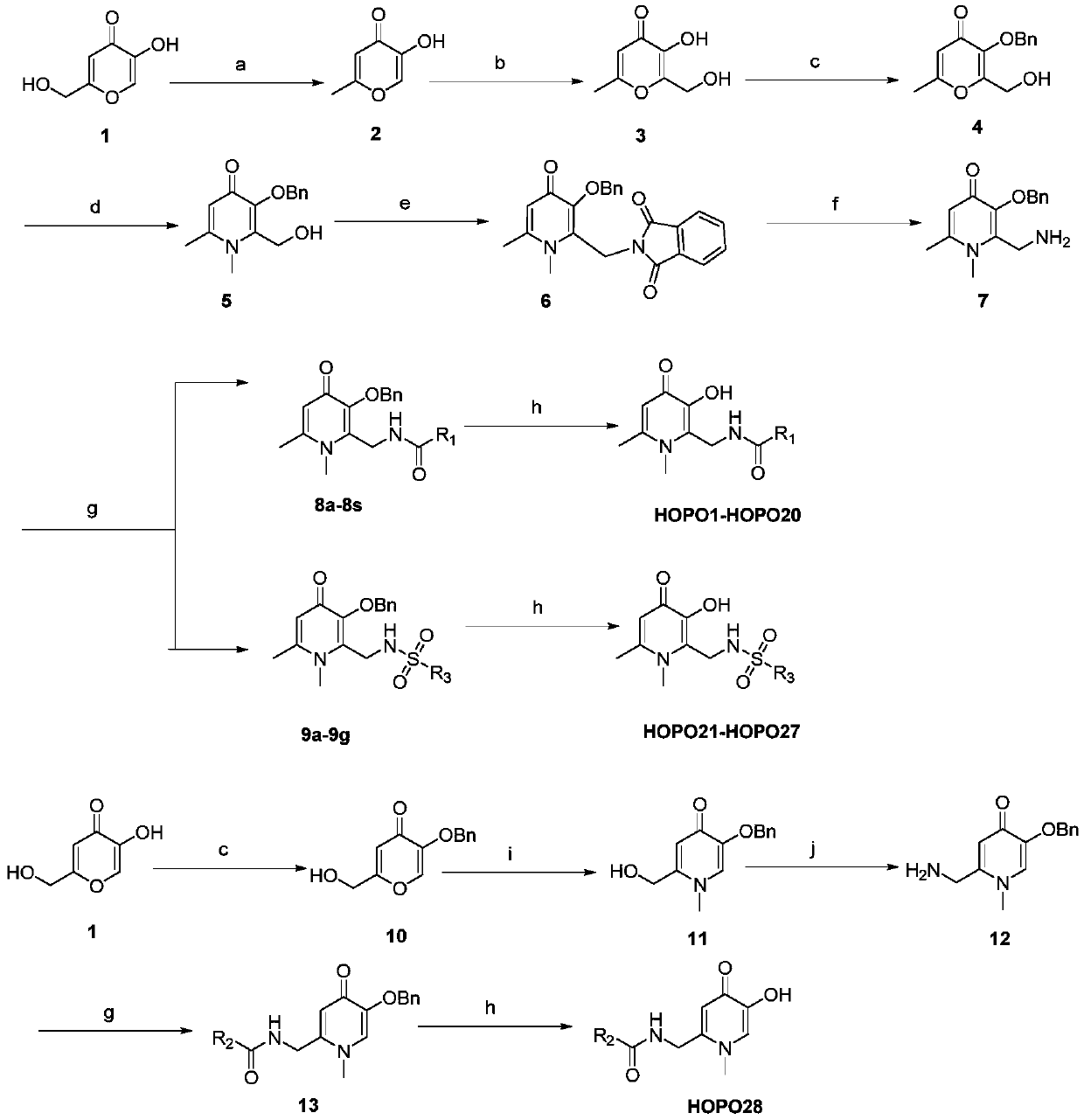
[0058] Embodiment 1: the preparation of 5-hydroxyl-2-methyl-4-pyrone (2)
[0059]
[0060] Add kojic acid (50g, 0.35mol) to a 500mL two-necked bottle, add 127mL of SOCl 2 (5eq), stirred at room temperature for 1-2 hours, after the reaction, a yellow solid was obtained, added petroleum ether and stirred for 10-30 minutes, filtered to obtain 56g of filter cake; filter cake (56g, 0.35mol) was dissolved in 200mL water, heated After reaching 50-55°C, add zinc powder (45.5g, 0.70mol), add 105mL of concentrated brine dropwise at 0.5-5mL / min, control the temperature at 70°C-80°C, and react at 70-80°C for 3-5 Hour. Stop the reaction, remove the insoluble matter by hot filtration, extract the filtrate 3 times with dichloromethane, dry the collected dichloromethane with anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain the crude product, which is recrystallized with isopropanol / petroleum ether , to obtain white solid compound 2 (37g, 84.1%), melting ...
Embodiment 2
[0062] Embodiment 2: Preparation of 3-hydroxyl-2-(hydroxymethyl)-6-methyl-4-pyrone (3)
[0063]
[0064] Put compound 2 (37g, 0.29mol) in a reaction flask, add NaOH (13g, 0.32mol), add 200mL of water, add 27mL of 35%-38% formaldehyde solution, and stir overnight at room temperature. After the reaction is completed, use 36% concentrated hydrochloric acid to adjust the pH to 1, then cool to 5-0°C and a large amount of solids will precipitate. When the solids no longer precipitate, filter the filter cake and dry the filter cake to obtain white solid compound 3 (42.7g, 93.2%), melting point: 158.3-159.2°C.
[0065] 1 H NMR (300MHz, DMSO-d 6 )δ2.26(s,3H,CH 3 ),4.39(s,2H,CH 2 OH),5.36(brs,1H,CH 2 OH), 6.22(s, 1H, C=CH), 8.87(s, 1H, C=C-OH); 13 C NMR (75MHz, DMSO-d 6 )δ174.36, 165.08, 149.88, 141.73, 111.60, 55.45, 19.75
Embodiment 3
[0066] Example 3: Preparation of 3-(benzyloxy)-2-(hydroxymethyl)-6-methyl-4-pyrone (4)
[0067]
[0068] Compound 3 (42.7g, 0.27mol) was dissolved in 150mL methanol, NaOH (12g, 0.30mol) was added, heated to 75-80°C and refluxed, and benzyl bromide (46.2g, 0.27mol) was added dropwise at 0.5-2mL / min ), reflux reaction at 75-80°C overnight; after the reaction was completed, concentrate under pressure to obtain a residue, dissolve the residue in 200mL water, extract three times with dichloromethane, and wash the collected dichloromethane twice with 5% aqueous sodium hydroxide solution. Washed twice with saturated brine, collected the organic phase, dried over anhydrous sodium sulfate, filtered the crude product concentrated under reduced pressure, and recrystallized the crude product with dichloromethane / petroleum ether to obtain white solid compound 4 (56.6g, 85.2%) , Melting point: 113.2-114.0°C.
[0069] 1 H NMR (300MHz, DMSO-d 6 )δ2.26(s,3H,CH 3 ),4.28(d,2H,J=6.0Hz,CH ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com