Free radicalized ethylene-maleic anhydride copolymer and synthesis method thereof
A technology of maleic anhydride and synthesis method, which is applied in the field of free radical ethylene maleic anhydride copolymer and its synthesis, can solve the problems such as complicated reaction process, and achieve the effect of simple reaction, high molecular weight and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The invention provides a kind of synthetic method of radicalized ethylene maleic anhydride copolymer, comprises the following steps:
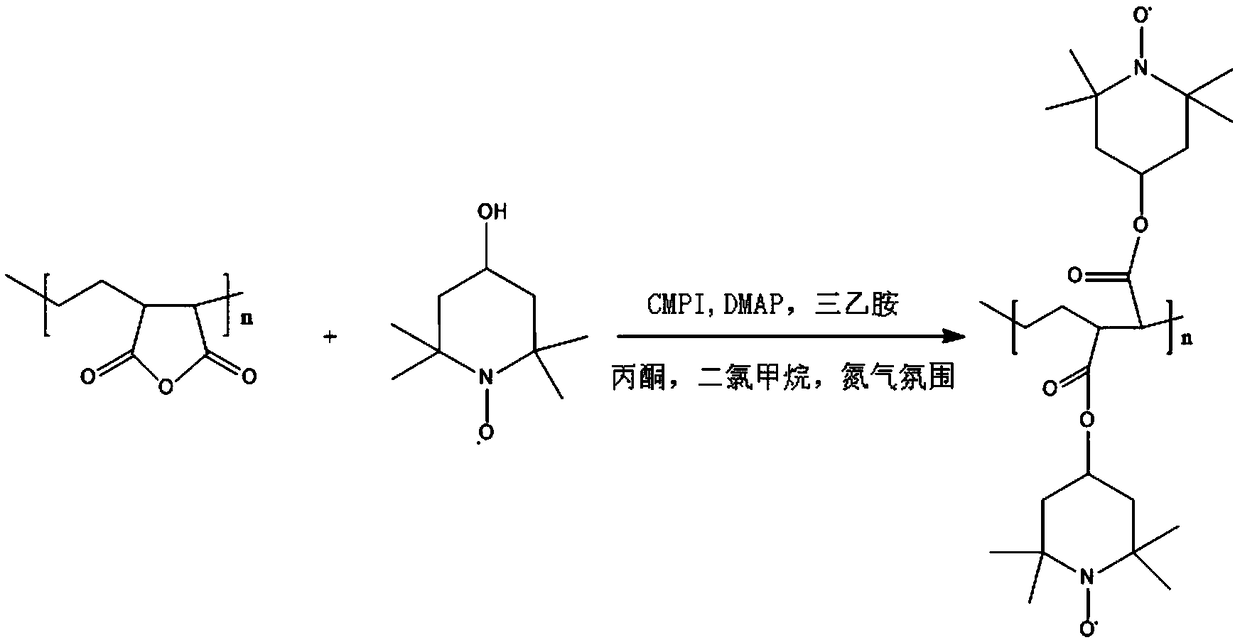
[0031] Under the catalysis of the catalytic system, the ethylene-maleic anhydride copolymer and 4-hydroxyl-2,2,6,6-tetramethylpiperidine nitroxide radicals undergo an esterification reaction in a polar organic solvent to obtain Free radicalized ethylene maleic anhydride copolymer.
[0032] The present invention preferably carries out esterification reaction after mixing ethylene-maleic anhydride copolymer, 4-hydroxy-2,2,6,6-tetramethylpiperidine nitroxide free radical, catalytic system and polar organic solvent to obtain free Hydroxylated ethylene maleic anhydride copolymer. The present invention has no special limitation on the mixing mode of ethylene-maleic anhydride copolymer, 4-hydroxyl-2,2,6,6-tetramethylpiperidine nitroxide radical, catalytic system and polar organic solvent. A mixing method well known to those skilled in the art...
Embodiment 1
[0054] Solvent volume ratio:
[0055] Acetone: 70mL;
[0056] Ultra-dry dichloromethane: 10mL;
[0057] The proportion of reaction raw materials:
[0058] Commercial ethylene maleic anhydride copolymer (Mw=10,0000-45,0000) (PEM) 6mmol;
[0059] 4-Hydroxy-2,2,6,6-tetramethylpiperidine nitroxide radical (TEMPO-OH) 12mmol;
[0060] Catalyst 4-dimethylaminopyridine (DMAP): 3.6 mmol;
[0061] Acylating reagent 2-chloro-1-methylpyridinium iodide (CMPI): 6mmol;
[0062] Organic base triethylamine: 12mmol;
[0063] According to the proportioning ratio of substances, 6mmol ethylene maleic anhydride copolymer and 12mmol 4-hydroxyl-2,2,6,6-tetramethylpiperidine nitroxide radicals were completely dissolved in 40mL acetone; 3.6mmol DMAP was dissolved Add 30mL of acetone, and drop in the solution obtained above; add 18mmol of triethylamine, 6mmol of CMPI and 10mL of dichloromethane in sequence, and stir the reaction at room temperature in a nitrogen atmosphere; after reacting for 1 da...
Embodiment 2
[0065] Solvent volume ratio:
[0066] Acetone: 60mL;
[0067] Ultra-dry dichloromethane: 20mL;
[0068] The proportion of reaction raw materials
[0069] Commercial ethylene maleic anhydride copolymer (Mw=10,0000-45,0000) (PEM) 6mmol;
[0070] 4-Hydroxy-2,2,6,6-tetramethylpiperidine nitroxide radical (TEMPO-OH) 12mmol;
[0071] Catalyst 4-dimethylaminopyridine (DMAP): 3.6 mmol;
[0072] Acylating reagent 2-chloro-1-methylpyridinium iodide (CMPI) 6mmol;
[0073] Organic base triethylamine 12mmol;
[0074] According to the proportioning ratio of substances, 6mmol ethylene maleic anhydride copolymer and 12mmol 4-hydroxy
[0075] -2,2,6,6-Tetramethylpiperidine nitroxide completely dissolved in 40mL of acetone; 3.6mmol of DMAP was dissolved in 20mL of acetone, and dropped into the aforementioned solution; 18mmol of triethylamine, 6mmol of CMPI and 20mL dichloromethane, and stirred at room temperature in a nitrogen atmosphere; after 1 day of reaction, the mixed solvent in the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com