Application of cinchonain Ib to preparation of medicament for preventing and treating rheumatoid arthritis
A technology for rheumatoid and arthritis, which is applied in the application field of cinchonine Ib in the preparation of drugs for the prevention and treatment of rheumatoid arthritis, and can solve the problems of toxic side effects affecting patient compliance, high price, and difficulty in popularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Extraction: 5kg fresh Fuji apple pomace black 40L water 80 ℃ reflux extraction 3 times, the extraction time is 3, 2, 1 hour respectively, filter, the mesh number of the filter is 120, the extracts are combined and filtered, filtered The mesh number of the net is 100, and the supernatant is obtained; or select 200g of commercially available apple polyphenols (provided by Tianjin Jianfeng Natural Products Research and Development Co., Ltd., polyphenol content 80%), add 2000mL of water at room temperature and stir to dissolve, set aside ;
[0022] (2) Adsorption and separation of macroporous resin: Adsorb the apple polyphenol solution in (1) through HP20 macroporous resin, and use water and ethanol aqueous solution gradients with volume percentage concentrations of 10%, 30%, 50%, 70%, and 95%. For elution, each half of the column bed volume is used as a receiving volume, and the components rich in cinchonaine Ib are collected by thin-layer chromatography combined with ...
Embodiment 2
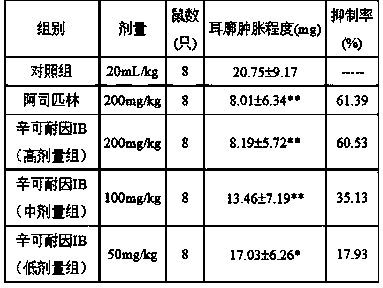
[0031] The maximum tolerated dose test and anti-inflammatory activity test of cinchonine Ib.
[0032] 1. Test materials: Animals: ICR mice, 18~22g, both male and female; Wistar rats, weighing 160~190g, male, provided by Beijing Experimental Animal Research Center.
[0033] 2. Reagents: Cinconate Ib (made in the laboratory according to the method in the example, with a purity of 95%); aspirin was purchased from Hunan Yongke Light Industrial Products Import and Export Co., Ltd.; carrageenan was purchased from China Lianxing Industrial Co., Ltd.; Analytical balance, vortex mixer.
[0034] 3. Test method: (1) Maximum tolerance test: divide 40 ICR mice into two groups, group 1, normal water control group; group 2, cinchonaine Ib group. There were 20 mice in each group, half male and half male. After fasting for 12 hours, intragastric administration of cinchonaine Ib with a maximum concentration of 0.2 g / mL was administered to the mice once every 4 hours. Administer 3 times a da...
Embodiment 3
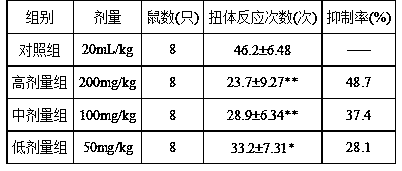
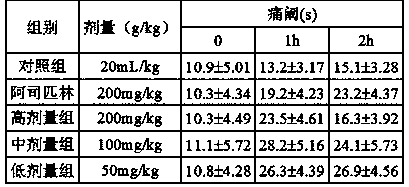
[0046] Analgesic activity test of cinchonaine Ib.
[0047] 1. Test materials: Animals: ICR mice, 18-22g, both male and female; provided by Beijing Experimental Animal Research Center.
[0048] 2. Reagents: Cinchonaine Ib (self-made in the laboratory, Cinchonaine Ib content 95%); Aspirin was purchased from Hunan Yongke Light Industrial Products Import and Export Co., Ltd.; acetic acid was of analytical grade, electronic analytical balance, vortex mixer.
[0049] 3. Test methods: (1) Acetic acid writhing test: 40 mice, half male and half male, were randomly divided into 5 groups, respectively administered with drugs or the same amount of water, and 1 hour after the drug, each mouse was intraperitoneally injected with 0.7% HAC normal saline solution 0.2 ml, observe the number of writhing reactions in mice within 20 min after HAC injection, and calculate the analgesic inhibition percentage.
[0050]
[0051] (2) Mouse hot plate test: test according to Zhao Yi’s method. Fema...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com