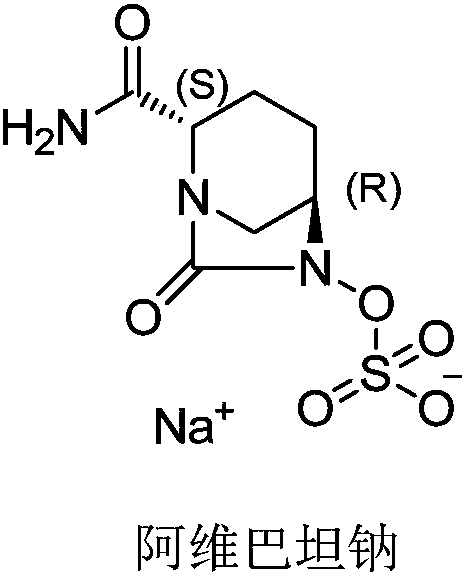
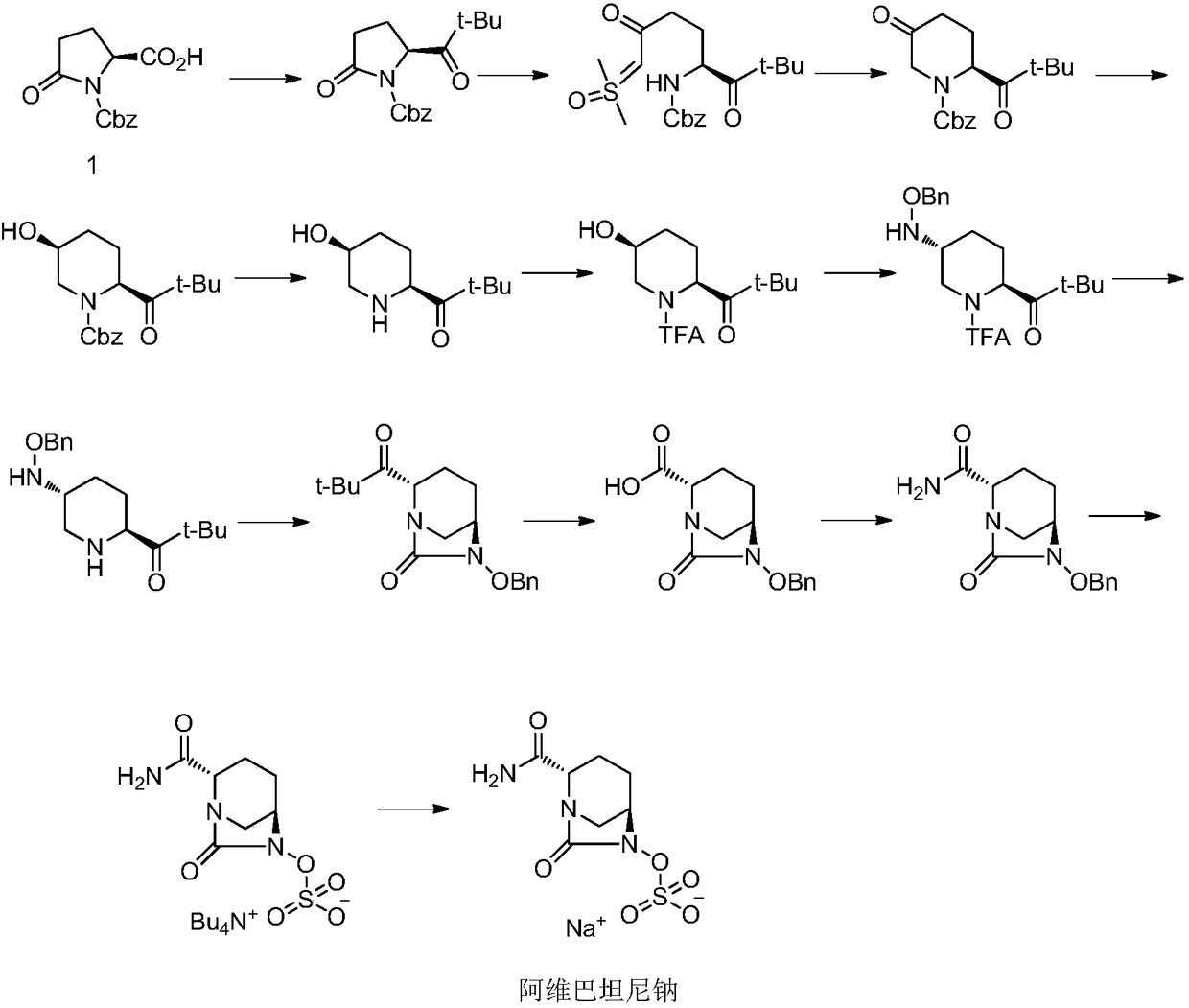
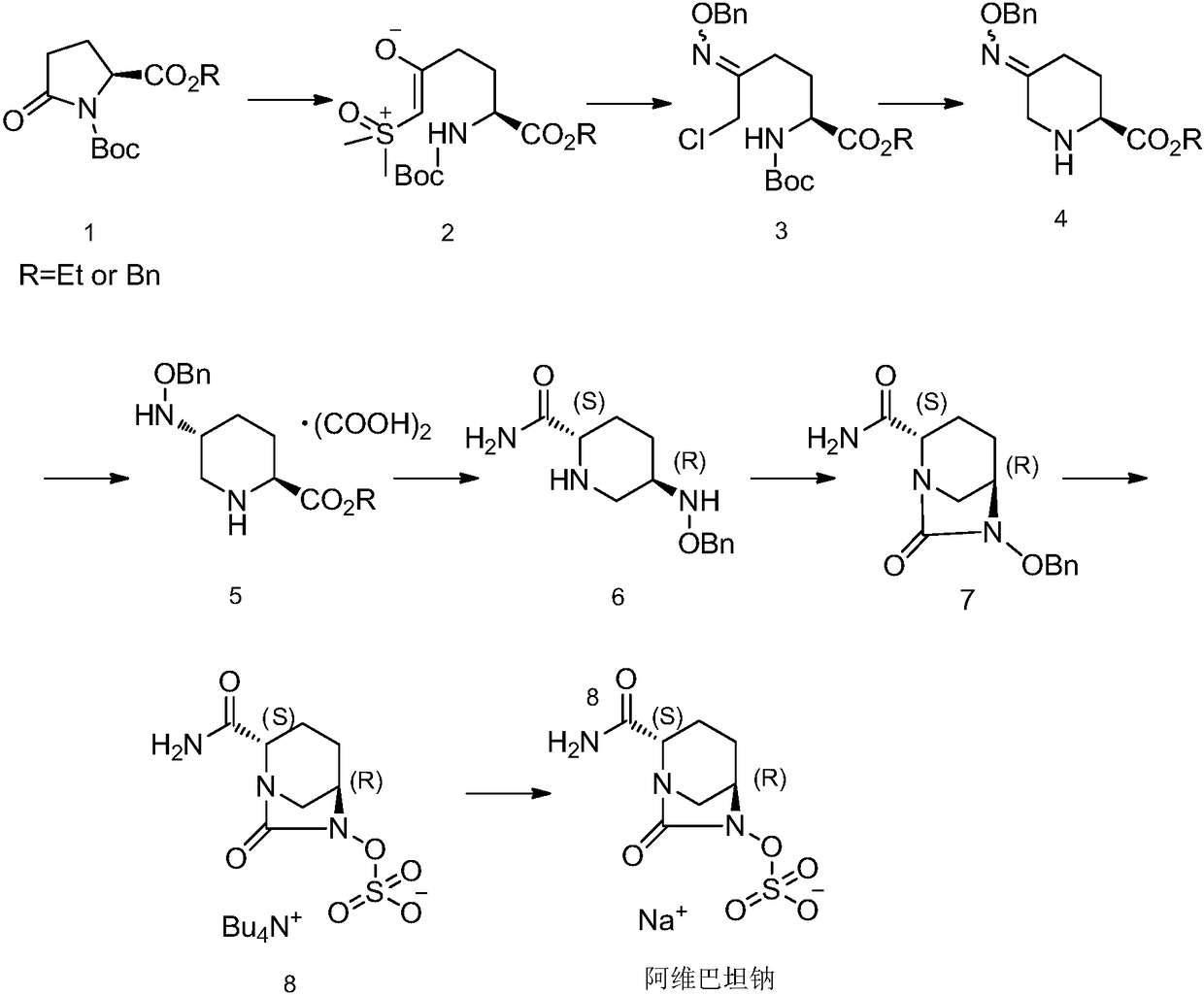
Avibactam sodium synthesis method
A technology of avibactam sodium and a synthesis method, which is applied in the field of medicinal chemistry synthesis, can solve the problems of limited industrialization potential, expensive reaction reagents, harsh reaction step conditions, etc., and achieves reduction of synthesis difficulty and reduction of high-risk and high-toxicity reagents. Use and reaction conditions are mild and easy to control the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0043] The present invention will be further described in conjunction with specific examples, but the content of the present invention is not limited by the examples.
[0044] One, the preparation of ethyl 5-hydroxy-2-piperidinecarboxylate (2)
[0045]
[0046] Add ethyl 5-hydroxy-2-pyridinecarboxylate (1) (25g, 149.6mmol), rhodium carbon (2.5g, 10%Rh / C) and ethanol (150ml) into the hydrogenation kettle and stir to dissolve, pressurize the hydrogen to 200psi Reaction 12h. The solvent was removed by rotary evaporation to obtain a crude product (25.3 g) of ethyl 5-hydroxy-2-piperidinecarboxylate (2), which was directly subjected to the next reaction without purification.
[0047] Two, the preparation of (2S,5S)-5-hydroxyl-2-piperidinecarboxylic acid ethyl ester (3)
[0048]
[0049] Add crude 5-hydroxy-2-piperidinecarboxylate ethyl ester (2) (25.3 g, 146 mmol) and potassium phosphate buffer solution (2.53 L, 0.1 M, pH=8) into a 5 L single-necked flask to adjust the pH va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com