Electrochemical sensor for sensitively detecting heavy metal cadmium ions and preparation method of electrochemical sensor
A technology for sensitive detection of cadmium ions, applied in the field of trace heavy metal electrochemical sensors, can solve the problems of complex operation of equipment, real-time online detection of heavy metal cadmium ions, long time consumption of sample preparation, narrow detection linear range, etc., to improve electrocatalytic performance , Conducive to the effect of enrichment and detection with a wide linear range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
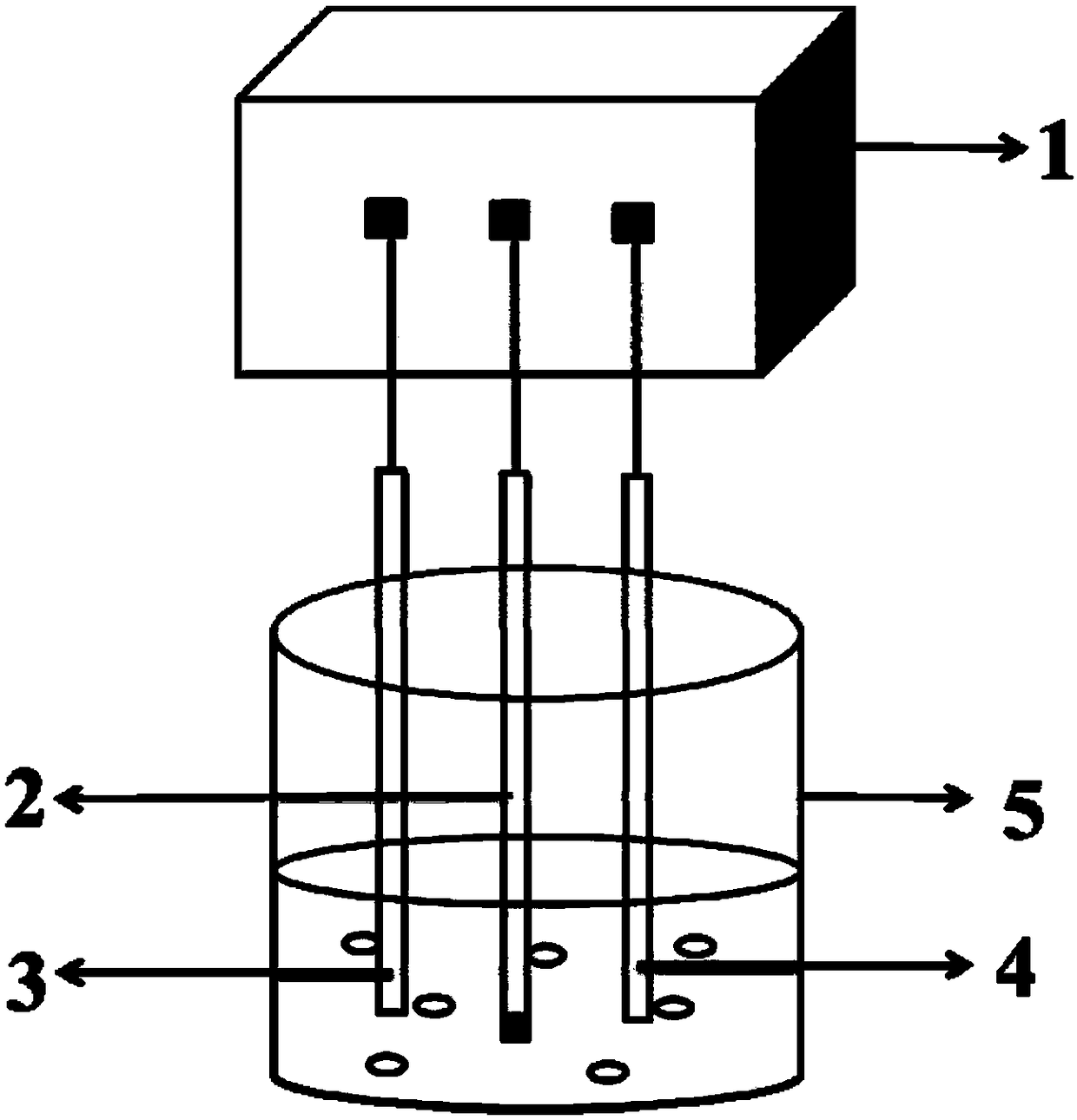
[0036] Preparation of a glassy carbon electrode modified with nitrogen and sulfur co-doped graphite porous carbon / Nafion / bismuth film
[0037] Preparation of Nitrogen and Sulfur Co-doped Graphite Porous Carbon
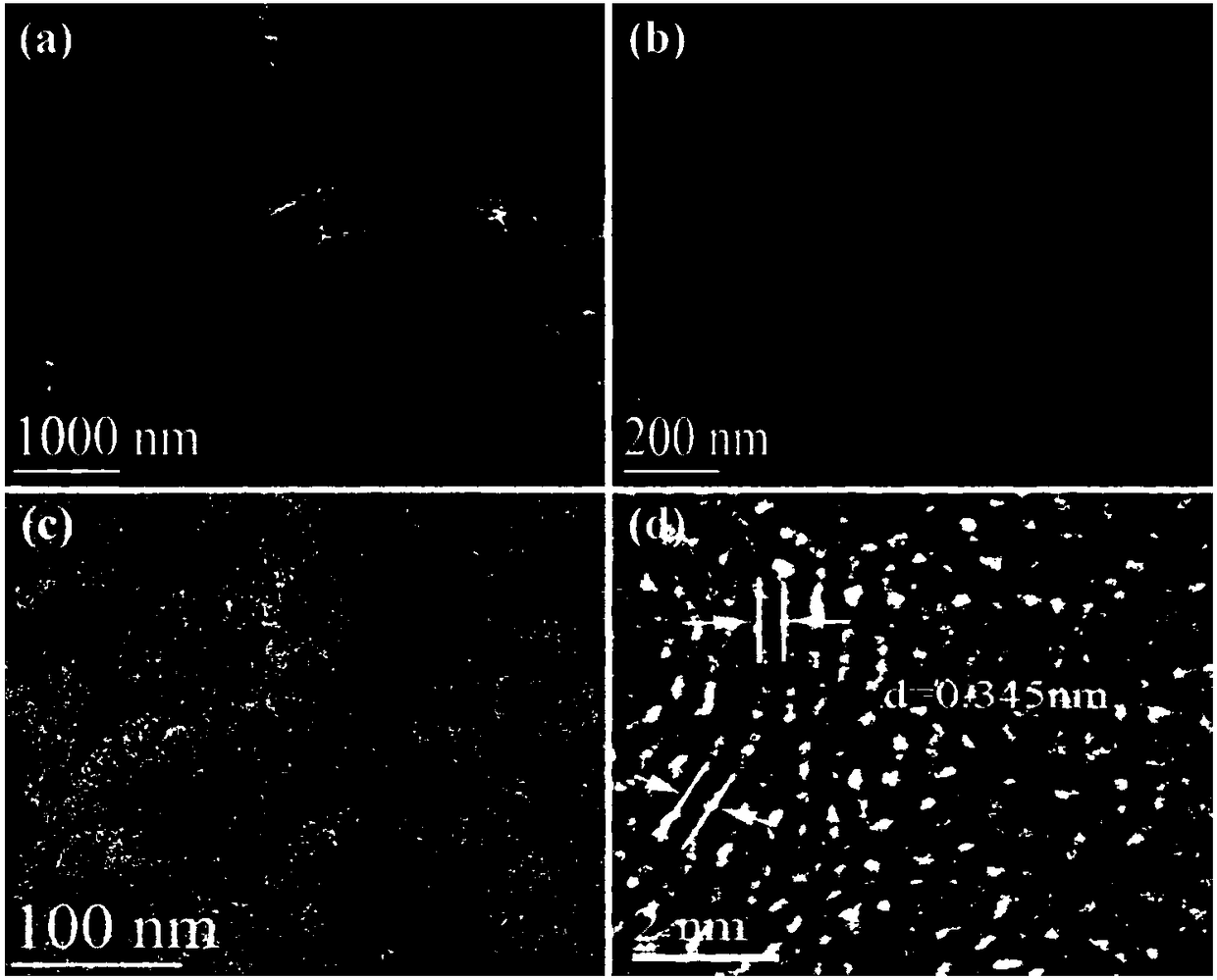
[0038] Mix 7.5 ml of ammonia water, 14 ml of deionized water and 300 ml of ethanol together, stir at room temperature for 30 minutes, and then slowly add a solution containing 15 ml of ethanol and 15 ml of ethyl orthosilicate to The above mixed solution. The resulting mixed solution is stirred for 6 hours to prepare uniform silica microspheres. 7.5 g of p-this diamine and 15 ml of sulfuric acid were added to the above colloidal solution and reacted for a further 36 hours. Subsequently, the final powdery substance can be obtained by centrifugation, drying at 80°C, and carbonization at 900°C in an air atmosphere. Finally, by soaking the above powder with hydrofluoric acid to remove the silica template, the nitrogen-sulfur co-doped graphite porous carbon can be obtained.
[...
Embodiment 2
[0044] Optimization of the concentration of nitrogen and sulfur co-doped graphite porous carbon dispersion.
[0045] Example 1 was repeated, and the glassy carbon electrode was modified with the modification solution obtained in Example 1 with a concentration of 0, 1, 2, and 3 mg / mL. The electrode peak current first increases and then decreases with the increase of the concentration of the modification solution. The optimal concentration of the modification solution is 1 mg / mL.
Embodiment 3
[0047] Optimization of Nafion quality score.
[0048] Repeat Example 2, and the mass fractions of Nafion used were 0, 0.1, 0.2 wt.%, respectively. When the mass fraction of Nafion increases from 0 to 0.1 wt.%, the peak current increases, but when the mass fraction of Nafion is further increased, the current decreases. The optimal mass fraction of Nafion is 0.1wt.%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com