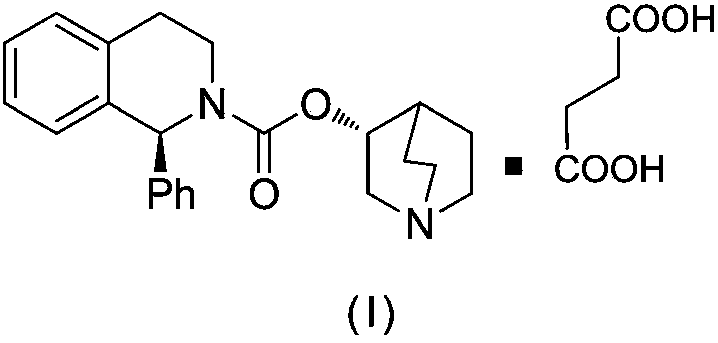
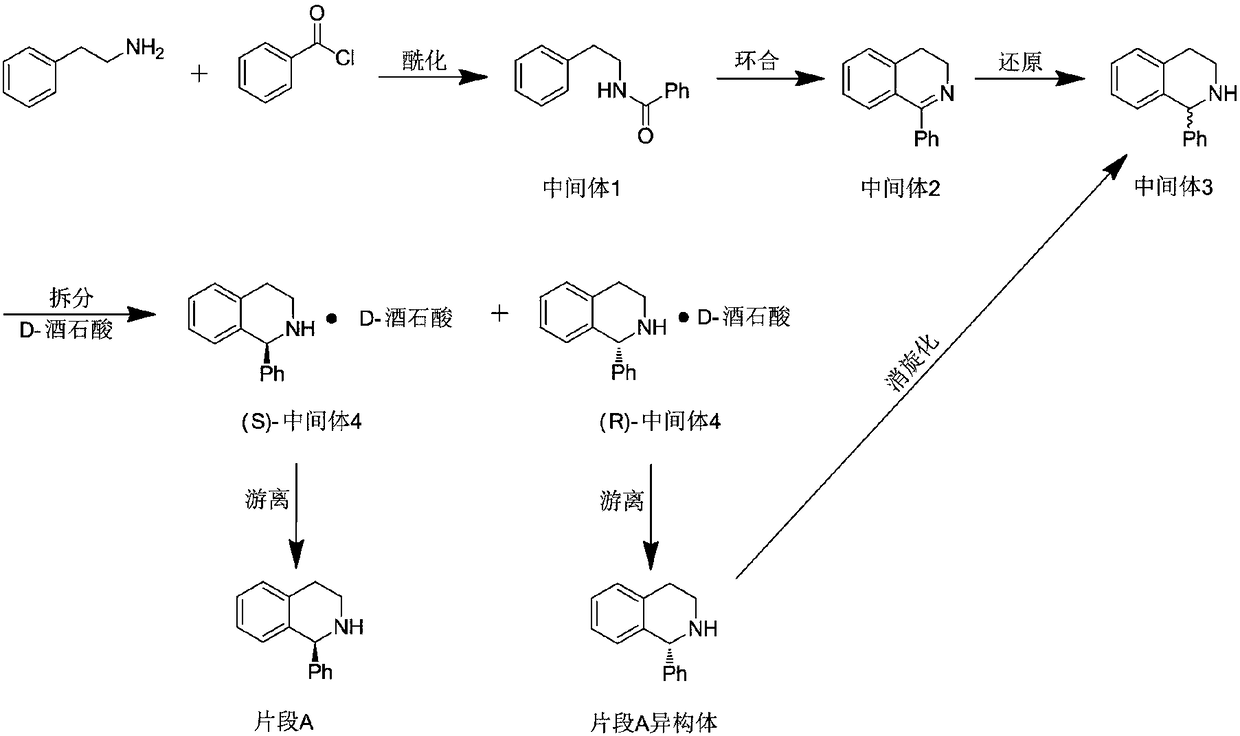
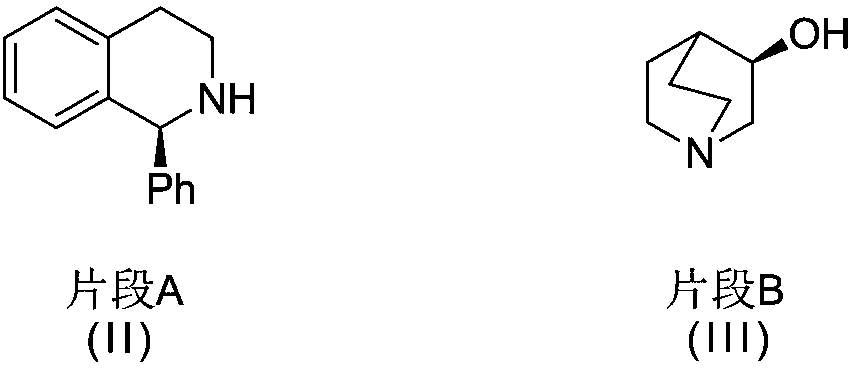Solifenacin succinate raw medicine synthesis process
A technology for the synthesis of solifenacin succinate, applied in the directions of organic chemistry, organic chemistry methods, etc., can solve the problems of no azeotrope relationship, unsatisfactory, affecting the optical purity of the final product segment B, etc., to simplify the operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: In a 5L four-necked bottle, add 113.8g of 2-phenylethylamine, 2.2L of n-heptane, and aqueous NaOH solution (47.8gNaOH is dissolved in 200mL of water), stir mechanically, and keep it in an ice-water bath at -5 to 10 Below ℃, 120g of benzoyl chloride was added dropwise. After the dropwise addition, the mixture was stirred and reacted at 20-25℃ for 3h, filtered, washed with water, and dried to obtain 188g of white solid with a yield of about 98% (based on benzoyl chloride).
Embodiment 2
[0100] Embodiment 2: In the four-neck bottle of 5L, add 2-phenethylamine 108.2g, n-heptane 1.6L, Na 2 CO 3 Aqueous solution (90.1g Na 2 CO 3 dissolved in 200mL water), mechanically stirred, ice-water bath, kept below -5 ~ 10 ℃, dropwise added 120g of benzoyl chloride, after the dropwise addition, continued to stir and react at 20 ~ 25 ℃ for 3h, filtered, washed with water, dried to obtain a white solid 185.8 g, the yield is about 97% (based on benzoyl chloride).
[0101] Fragment A second step: cyclization
[0102] Add 191.7g of intermediate 1 and 2.5L of toluene to a 5L four-neck bottle, stir and heat to 70°C, then add 241.3g of phosphorus pentoxide and 1173g of phosphorus oxychloride in sequence, heat to reflux for 8 hours, and recover by distillation under reduced pressure POCl 3 and xylene, the residual oil was added to ice water, stirred vigorously, and NaOH aqueous solution was added to adjust the pH to 8, and extracted with dichloromethane (1000ml×3). The organic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com