Detection reagent card and kit
A technology for detecting reagents and reagent cards, which is applied to biochemical equipment and methods, and microbial measurement/inspection. It can solve the problems of inconvenient self-testing by subjects, complicated operation, and poor user experience, and achieves improved sensitivity and simple operation. , the effect of relaxed detection conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
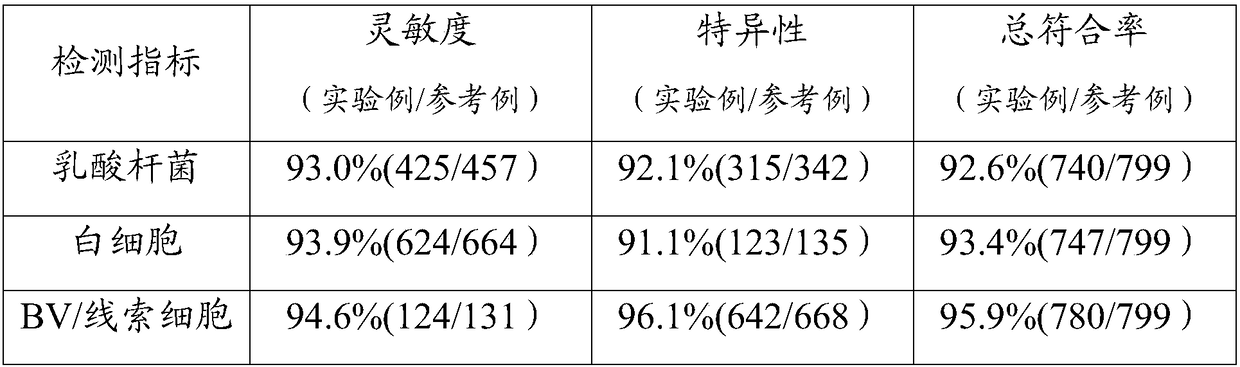
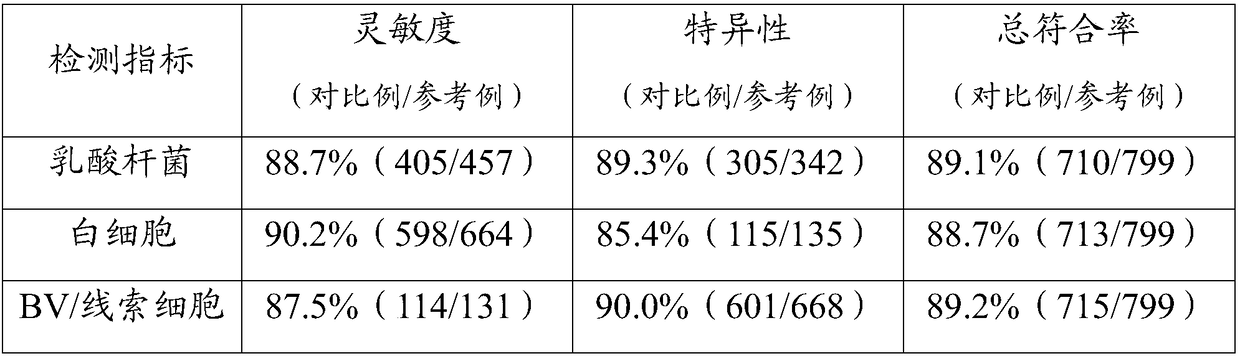
[0028] This embodiment provides a detection reagent card. The reagent card is provided with 4 detection grooves for detection, and reagent blocks are arranged in the detection grooves; the reagent blocks are obtained by drying the reaction solution after dripping into the reaction membrane.
[0029] The reaction solution is one of: sialidase reaction solution, hydrogen peroxide reaction solution, leukocyte esterase reaction solution or pH reaction solution.
[0030] Specifically, the reaction membrane may be one of filter paper, chromatography membrane or glass fiber membrane.
[0031] Preferably, the reactive membrane is filter paper.
[0032] The preparation method of the reagent card is as follows: cut the filter paper into small pieces with a diameter of 5mm, put it into the detection tank adapted on the reagent card, and mix the sialidase reaction solution, hydrogen oxide reaction solution, leukocyte esterase reaction solution and pH Each 5 μL of the reaction solution wa...
Embodiment 2
[0048] The difference between this embodiment and embodiment 1 is: the content of the reaction solution components of each detection index is different, specifically as follows:
[0049] 1) The sialidase reaction liquid contains the following components: 0.1g / L 5-bromo-4-chloro-3 indolyl acetylneuraminic acid sodium salt, 30g / L trehalose, 0.01mol / L 2-morpholine Sulfonic acid buffer, 0.01% wt nitro blue tetrazolium chloride, 0.05% wt NP-40, 0.1% bovine serum albumin, 1% wt sodium sulfate, pH 4.5.
[0050] 2) The hydrogen peroxide reaction liquid contains the following components: 10U / mL horseradish peroxidase, 5%wt glycerol, 0.05%wt bovine serum albumin, 10mmol / L phosphate buffer, 0.1mmol / L 4-amino Antipyrine, 0.5mmol / L 2-hydroxy-3-m-toluidine propanesulfonate sodium, 20mmol / L β-mercaptoethanol, pH7.2.
[0051] 3) Leukocyte esterase reaction solution contains the following components: 0.1g / L pyrrole ester, 0.05g / L 1-diazo-2-naphthol-4 sulfonate, 18%wt PVP K-30, 0.05mol / L Citr...
Embodiment 3
[0054] The difference between this embodiment and embodiment 1 is: the content of the reaction solution components of each detection index is different, specifically as follows:
[0055] 1) The sialidase reaction liquid contains the following components: 0.3g / L 5-bromo-4chloro-3 indolyl acetylneuraminic acid sodium salt, 60g / L trehalose, 0.5mol / L 2-morpholine Sulfonic acid buffer, 0.1% wt nitro blue tetrazolium chloride, 0.5% wt NP-40, 1% bovine serum albumin, 5% wt sodium sulfate, pH 5.5.
[0056] 2) The hydrogen peroxide reaction liquid comprises the following components: 50U / mL horseradish peroxidase, 20%wt glycerol, 2%wt bovine serum albumin, 200mmol / L phosphate buffer, 0.5mmol / L 4-amino Antipyridine, 2mmol / L 2-hydroxy-3-m-toluidine propanesulfonate sodium, 100mmol / L DTT, pH7.0.
[0057] 3) Leukocyte esterase reaction solution contains the following components: 0.5g / L pyrrole ester, 0.3g / L 1-diazo-2-naphthol-4 sulfonate, 30%wt PVP K-30, 0.2mol / L Citrate buffer, 5% wt dec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com