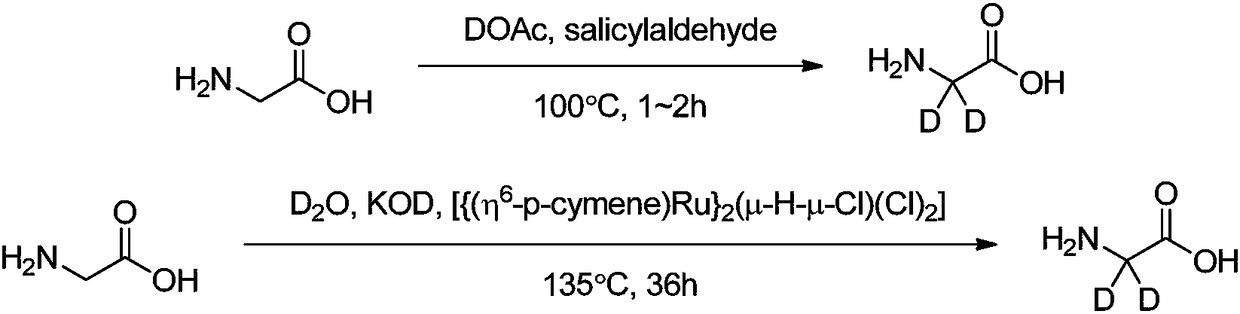
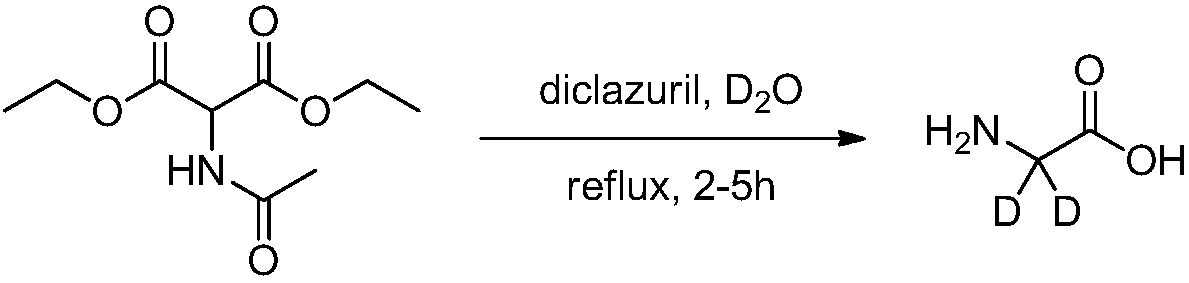
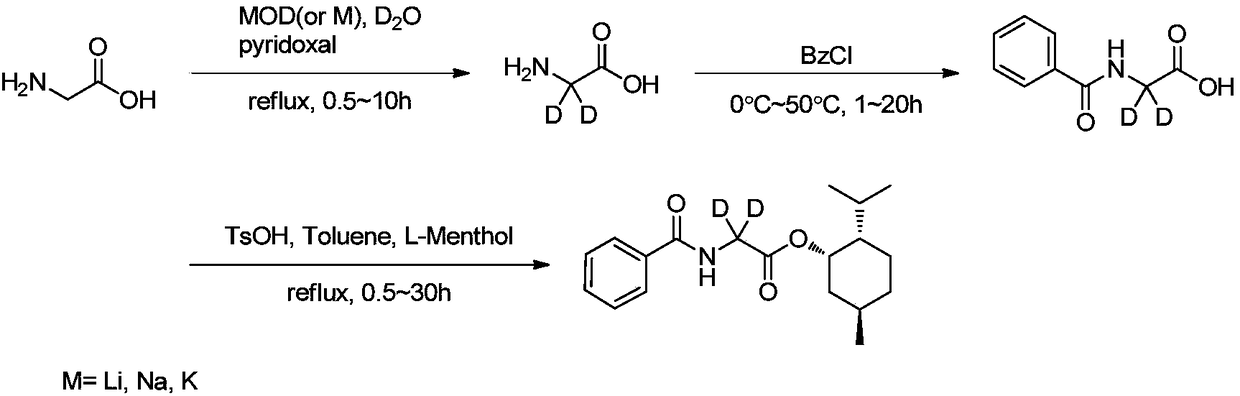
Synthesis methods of deuterine, hippuric acid-L-menthol ester (2,2-D2) and intermediates thereof
A 2-D2, synthetic method technology, applied in the direction of organic chemical methods, chemical instruments and methods, carboxylic acid amide preparation, etc., can solve the problems of harsh reaction conditions, strict equipment requirements, unfavorable preparation, etc., and achieve easy access to raw materials, equipment Corrosion reduction, easy to scale up the effect of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] After successively adding 1.0 g of glycine and 100 mg of pyridoxal hydrochloride into the single-mouth bottle, the oil pump was replaced three times under the protection of argon, and then 10 mL of 10% (mass percentage) deuterium deuterium oxide aqueous solution was added under an argon atmosphere, and the reaction was refluxed for 2 hours. . After the reaction is finished, cool, (filter with a needle filter), spin out about 50% of deuterium water in a vacuum to recover, add 10 mL of distilled water after cooling, add 2.2 mL of benzoyl chloride, stir at room temperature overnight, then add crude ice, use Concentrated hydrochloric acid was used to adjust the pH value of the reaction solution to 2-3. After cooling, a large amount of solids were precipitated, filtered, and the filter cake was washed with ice water. Filter cake is recrystallized with hot water again, separates out white crystal, filters and dries, obtains 2.2g hippuric acid (2,2-D 2 ) pure product, yield 9...
Embodiment 2
[0058] After adding 1.0g of glycine, 100mg of pyridoxal hydrochloride, and 420mg of lithium metal in the three-neck flask, the oil pump was replaced three times under the protection of argon. After the reaction of lithium and deuterium water was complete, the temperature was raised to reflux for 2.5 hours. After the reaction is finished, cool, (filter with a needle filter), spin out about 50% of deuterium water in a vacuum to recover, add 10 mL of distilled water after cooling, add 2.2 mL of benzoyl chloride, stir at room temperature overnight, then add crude ice, use Concentrated hydrochloric acid was used to adjust the pH value of the reaction solution to 2-3. After cooling, a large amount of solids were precipitated, filtered, and the filter cake was washed with ice water. Filter cake is recrystallized with hot water again, separates out white crystal, filters and dries, obtains 2.0g hippuric acid (2,2-D 2 ) pure product, yield 82.9%, product purity>99% (HPLC), LC-MS and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com