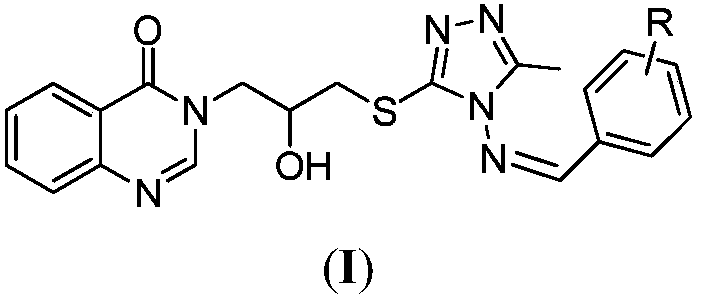
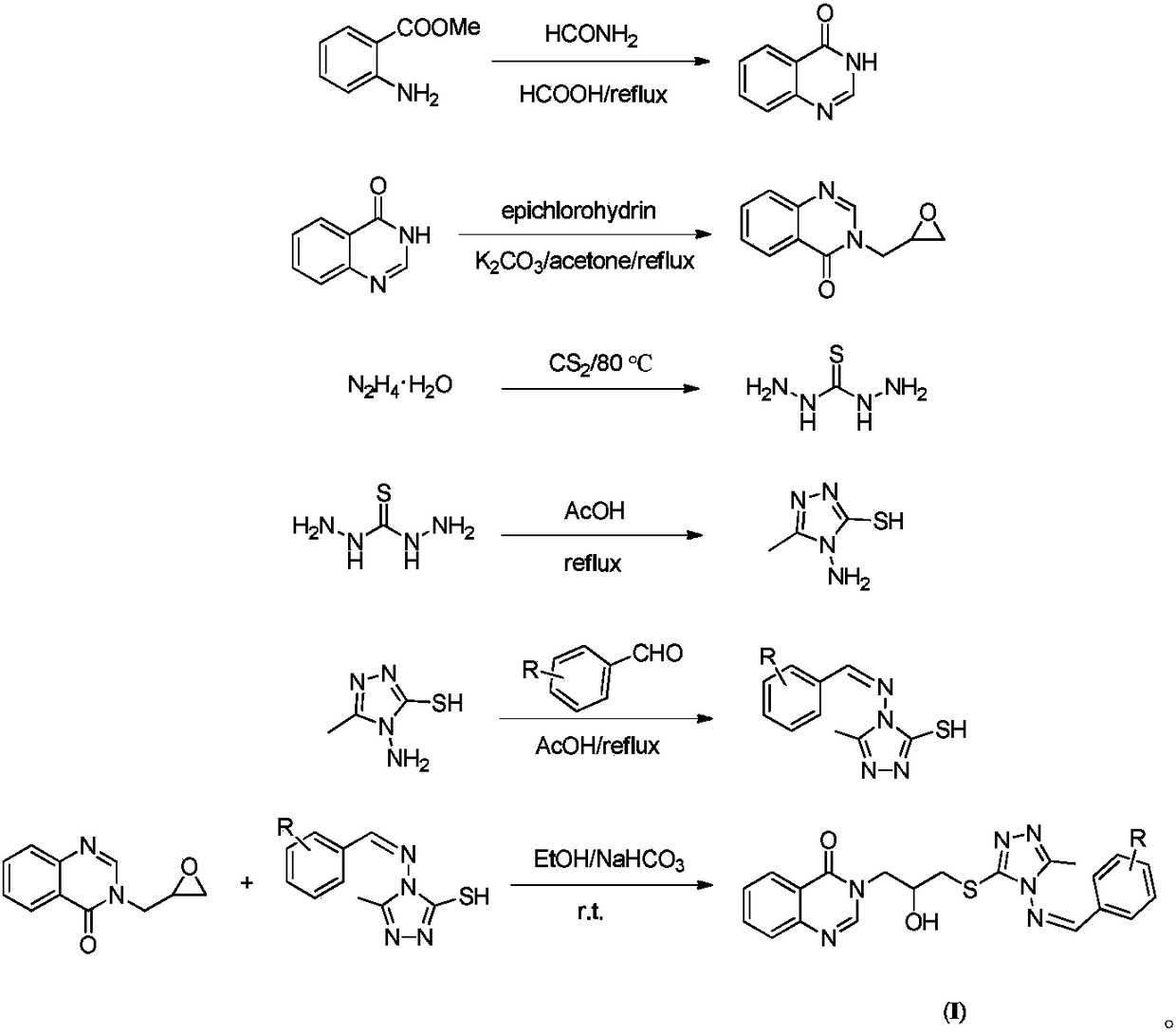
Compound for preparing crop pathogenic bacterium prevention and control medicament and preparation method thereof
A compound and synthesis method technology, applied in the field of chemistry, can solve the problems of reduced antibacterial efficacy, high toxicity of non-target organisms, and difficulty in degrading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
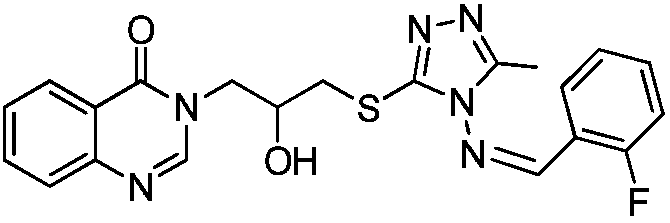
[0045] Example 1: Compound 3-(3-((4-((2-fluorobenzylidene)amino)-5-methyl-4H-1,2,4-triazol-3-yl)thio) -2-hydroxypropyl) quinazolin-4 (3H)-ketone synthesis:
[0046] (1) Preparation of quinazoline-4-one
[0047] Add 9.0g (59.5mmol) of methyl anthranilate, 13mL (327.3mmol) of formamide and 3mL (79.5mmol) of formic acid in sequence in a 100mL three-necked flask, heat up to 130-140°C for reflux for 6 hours, stop the reaction, and wait for the reaction After the liquid was cooled to room temperature, an appropriate amount of cold water was added. At this time, a large amount of white solids were precipitated, and the stirring was continued for 0.5 h. Suction filtration, washing with water, drying, and ethanol recrystallization gave 4.10 g of white flocs, with a yield of 47.2%.
[0048] (2) Preparation of 3-(2,3-epoxypropyl)quinazolin-4(3H)-one
[0049] Add 1.0g (6.84mmol) quinazolin-4-one, 50mL acetone and 1.2g (8.89mmol) potassium carbonate to a 100mL single-necked bottle, stir ...
Embodiment 2
[0059] Example 2: Compound 3-(3-((4-((3-fluorobenzylidene)amino)-5-methyl-4H-1,2,4-triazol-3-yl)thio) -2-hydroxypropyl) quinazolin-4 (3H)-ketone synthesis:
[0060] (1) Intermediates 4-((3-fluorobenzylidene)amino)-5-methyl-4H-1,2,4-triazole-3-thiol and 3-(2,3-epoxypropylene Base) the preparation of quinazoline-4 (3H)-ketone: synthesis steps and process conditions are the same as embodiment one (1~5);
[0061] (2) Target product 3-(3-((4-((3-fluorobenzylidene)amino)-5-methyl-4H-1,2,4-triazol-3-yl)thio) -2-hydroxypropyl) quinazolin-4 (3H)-one preparation (compound number A2):
[0062]
[0063] The synthesis steps and process conditions are the same as in Example 1 (6), except that m-fluorobenzaldehyde is used as a raw material to obtain a white solid with a yield of 88.6%. m.p.160~162℃, 1 H NMR (500MHz, DMSO-d 6 )δ:8.86(s,1H),8.19(s,1H),8.11(d,J=10.0Hz,1H),7.80-7.70(m,3H),7.63(d,J=5.0Hz,1H), 7.61-7.58(m,1H),7.52-7.45(m,2H),5.65(d,J=5.0Hz,1H),4.27-4.23(m,1H),4.07(s,1H),3...
Embodiment 3
[0064] Example 3: Compound 3-(3-((4-((4-fluorobenzylidene)amino)-5-methyl-4H-1,2,4-triazol-3-yl)thio) -2-hydroxypropyl) quinazolin-4 (3H)-ketone synthesis:
[0065] (1) Intermediates 4-((4-fluorobenzylidene)amino)-5-methyl-4H-1,2,4-triazole-3-thiol and 3-(2,3-epoxypropylene Base) the preparation of quinazoline-4 (3H)-ketone: synthesis steps and process conditions are the same as embodiment one (1~5);
[0066] (2) Target product 3-(3-((4-((4-fluorobenzylidene)amine)-5-methyl-4H-1,2,4-triazol-3-yl)thio) -2-hydroxypropyl) quinazolin-4 (3H)-one preparation (compound number A3):
[0067]
[0068] The synthesis steps and process conditions are the same as those in Example 1 (6), except that p-fluorobenzaldehyde is used as a raw material to obtain a white solid with a yield of 56.3%. m.p.209~210℃, 1 H NMR (500MHz, DMSO-d 6 )δ:8.85(s,1H),8.19(s,1H),8.11(d,J=10.0Hz,1H),8.00-7.97(m,2H),7.79(t,J=5.0Hz,1H), 7.63(d, J=5.0Hz, 1H), 7.51(d, J=5.0Hz, 1H), 7.40(d, J=5.0Hz, 2H), 5.65(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com