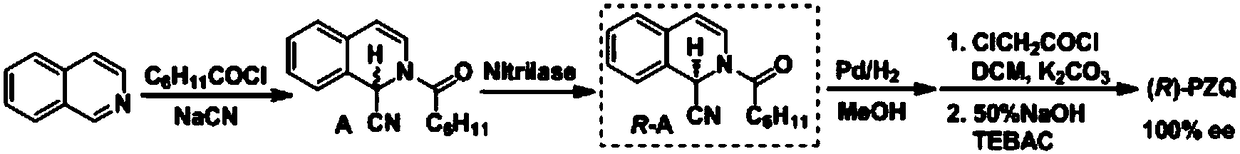
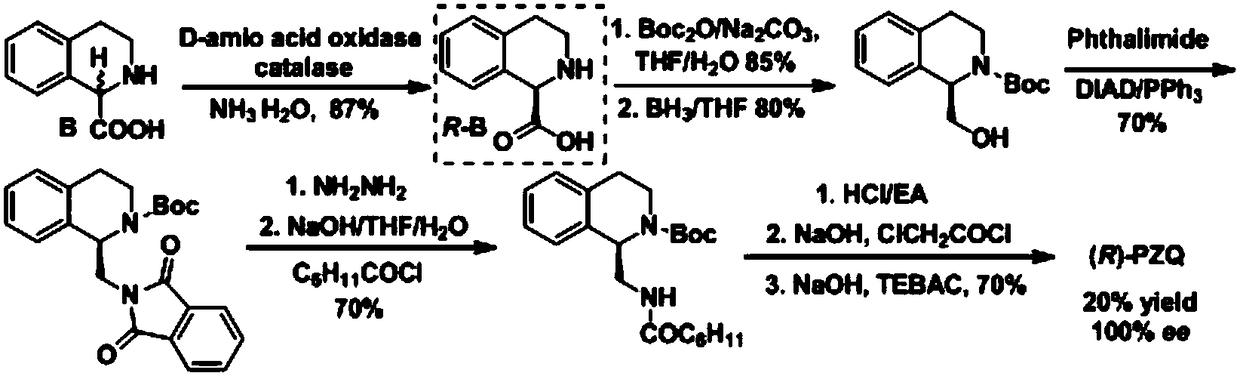
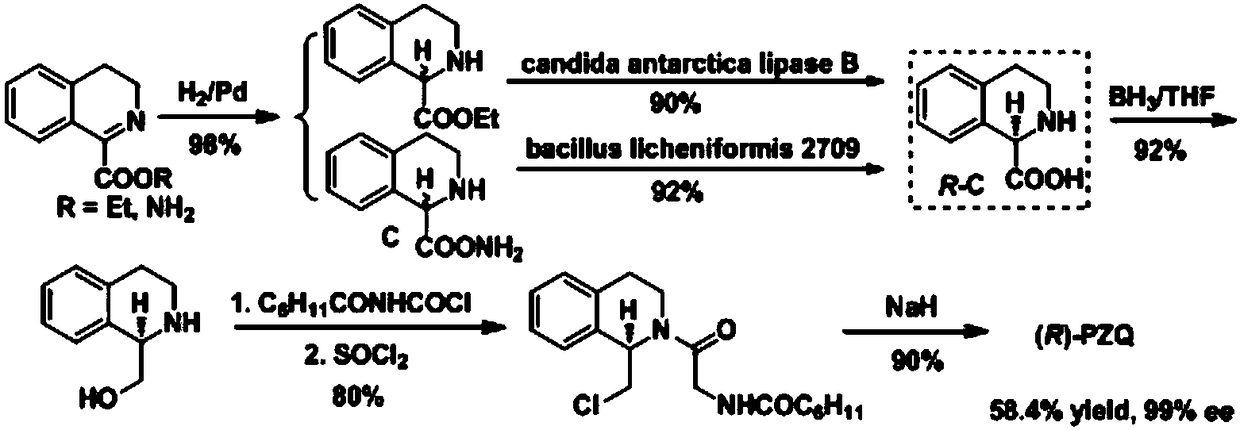
(R)-praziquantel intermediate and preparation method of (R)-praziquantel
A technology of praziquantel and intermediates, applied in the field of preparation of -praziquantel intermediates and -praziquantel, can solve the problems of using a large amount, high production cost, low efficiency, etc. good enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Preparation of (R)-1-nitromethyl-2-chloroacetyltetrahydroisoquinoline.
[0055]
example 1-1
[0056] Example 1-1: Dihydroisoquinoline (0.655g, 5mmol), nitromethane (0.915g, 15mmol), S-1,1'-bi-2-naphthol catalyst represented by formula (L4) ( 0.299g, 0.5mmol), triethylamine (1.518g, 15mmol), silica gel and sodium chloride (1.6g each) were sequentially added to a 25mL grinding jar, and then two 12mm stainless steel grinding balls were added. After sealing the grinding jar, place the grinding jar in a ball mill and grind at room temperature for 60 minutes at a grinding frequency of 10 Hz, then add chloroacetyl chloride (0.565 g, 5 mmol), and continue grinding for 30 minutes at the same grinding frequency. After the reaction was over, the reaction mixture was rinsed with petroleum ether and ethyl acetate at a volume ratio of 6:1 to obtain (R)-1-nitromethyl-2-chloroacetyltetrahydroisoquinoline 1.115g (yield: 83%, ee: 68%). Then, 10 mL of anhydrous methanol was used as a solvent for recrystallization at 40° C., and the mother liquor was collected and concentrated under redu...
example 1-2
[0058] Example 1-2: Dihydroisoquinoline (0.655g, 5mmol), nitromethane (1.068g, 17.5mmol), quinine-thiourea bifunctional catalyst represented by formula (L2) (0.149g, 0.25 mmol), tert-butylamine (0.365g, 7.5mmol), and neutral alumina (3.2g) were sequentially added to a 25mL grinding jar, and then three 10mm stainless steel grinding balls were added. After the grinding jar was sealed, the grinding jar was placed in a ball mill and ground at room temperature for 20 minutes at a grinding frequency of 30 Hz, then chloroacetyl chloride (1.129 g, 10 mmol) was added, and grinding was continued for 20 minutes at the same grinding frequency. After the reaction was finished, the reaction mixture was rinsed with petroleum ether and ethyl acetate at a volume ratio of 6:1 to obtain (R)-1-nitromethyl-2-chloroacetyl tetrahydroisoquinoline 1.102g (yield: 82%, ee: 72%). Recrystallization was carried out at 50° C. with 15 mL of methanol as a solvent, and the mother liquor was collected and conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com