Magnetic-particle chemiluminescent detection kit for 25-hydroxyvitamin D
A chemiluminescence detection and hydroxyvitamin technology, applied in the field of immunoassay, can solve the problems of instrument operation, high personnel quality requirements, inability to accurately quantify low-concentration samples, and high cost of instrument purchase and use. Accuracy, good capture effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 125
[0042] Example 1 Composition of 25-Hydroxyvitamin D Magnetic Particle Chemiluminescence Detection Kit
[0043] In this embodiment, the 25-hydroxyvitamin D magnetic particle chemiluminescent detection kit includes R1 reagent, R2 reagent, R3 reagent, magnetic separation reagent, calibrator solution series and quality control solution series; wherein,
[0044] In this embodiment, the R1 reagent is sodium hydroxide with a molar concentration of 0.2-0.5M; preferably, the R1 reagent is sodium hydroxide with a molar concentration of 0.5M.
[0045] In this embodiment, the R2 reagent includes alkaline phosphatase-labeled anti-25 hydroxyvitamin D monoclonal antibody, bovine serum albumin, animal serum, preservatives and buffer; alkaline phosphatase-labeled anti-25 hydroxyvitamin D monoclonal antibody The antibody concentration is 0.5-2.0 μg / mL; preferably, the concentration of alkaline phosphatase-labeled anti-25-hydroxyvitamin D monoclonal antibody is 1.0 μg / mL; the mass percentage con...
Embodiment 225
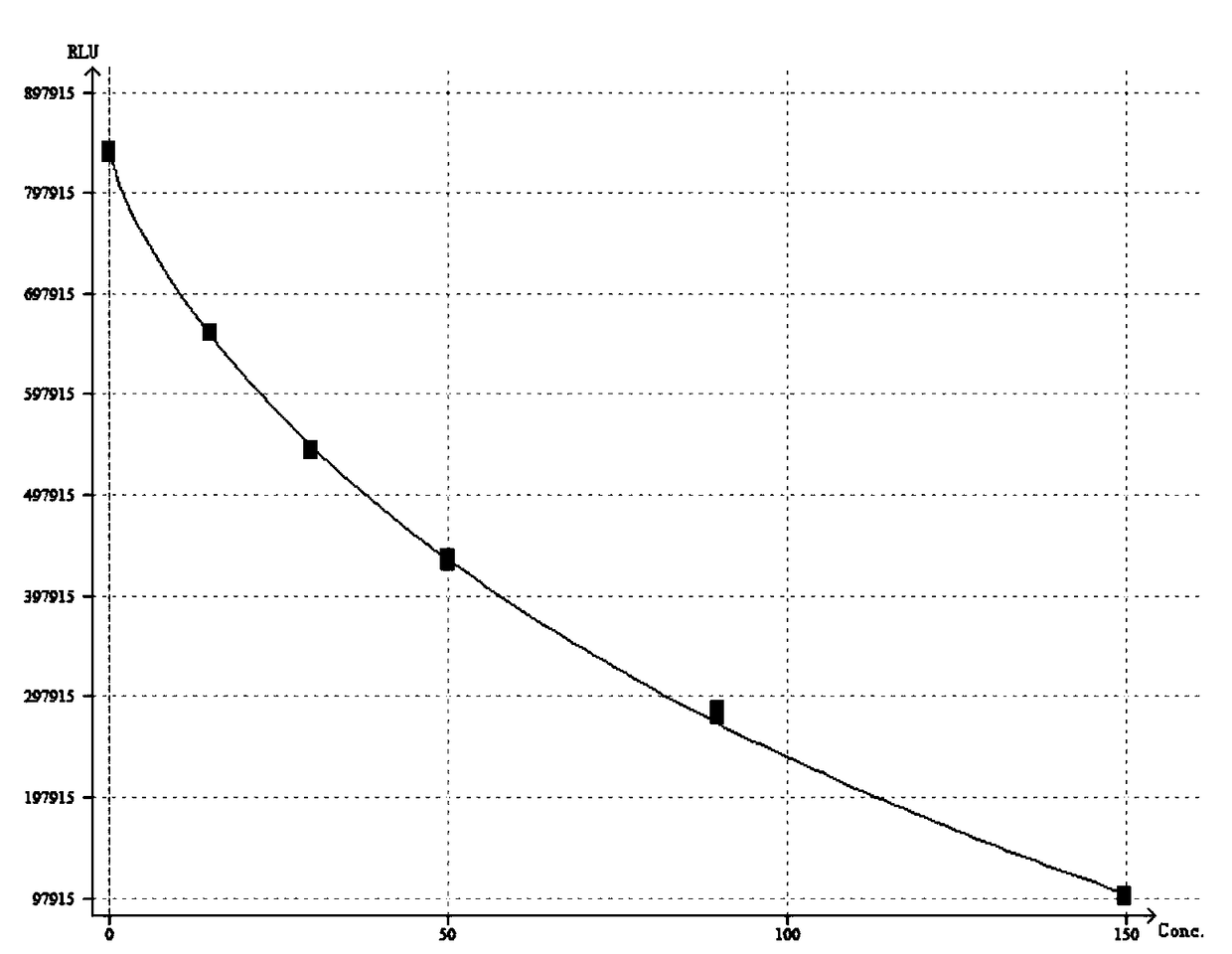
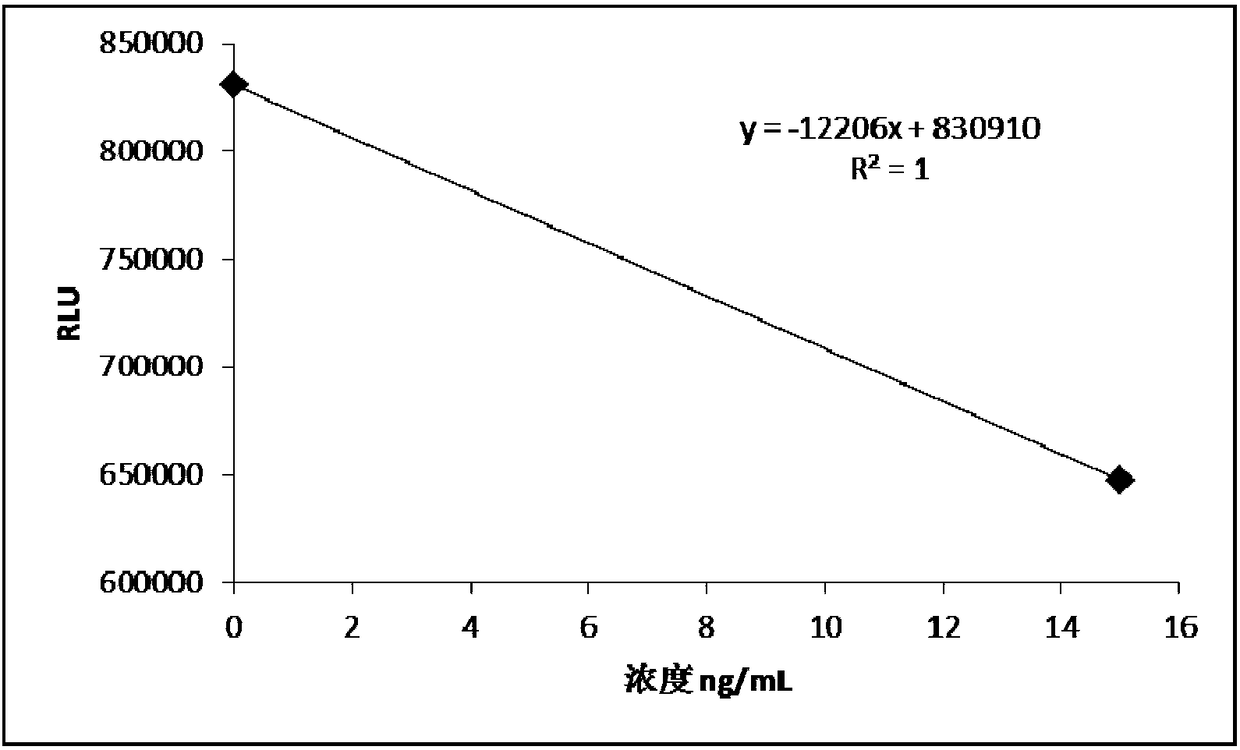
[0068] Example 2 Determination method of 25-hydroxyvitamin D magnetic particle chemiluminescence detection kit and standard curve drawing
[0069] Take the kit out of the storage condition and equilibrate to room temperature before using it for sample detection; mix thoroughly before the actual use of magnetic separation to ensure that the magnetic particles are evenly suspended, and do not use a magnetic stirrer for stirring; prepare the test tube and prepare it according to actual needs. mark.
[0070] Step 1. Take 2.0mL of venous blood into a glass test tube without adding anticoagulant, let it stand at room temperature, then centrifuge at 3000rpm for 5min, and take the supernatant;
[0071] Step 2. Take 10-50 μL of calibrator solution series, quality control solution series and collected serum samples into corresponding test tubes; the pipette head needs to be replaced before each sampling to avoid cross-contamination;
[0072] Step 3. Add 10-50 μL of R1 reagent to each t...
Embodiment 325
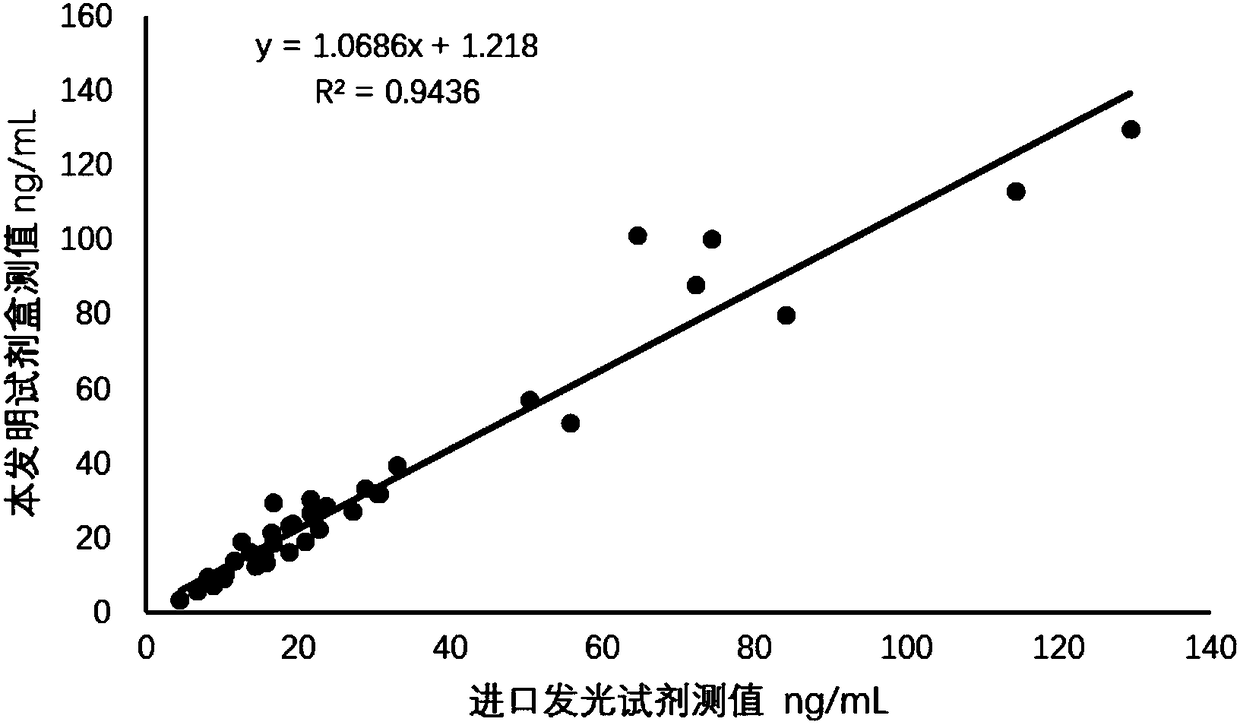
[0082] Example 3 Methodological Verification of 25-Hydroxyvitamin D Magnetic Particle Chemiluminescence Detection Kit
[0083] The test kit in Example 1 is tested according to the conventional manufacturing and testing procedures in the art, and the results are as follows:
[0084] 1. Determination of kit precision
[0085] 1.1 Intra-batch precision analysis
[0086] A batch of kits in Example 1 were used to measure high and low concentration quality control solution series respectively, and 10 wells were measured in parallel to obtain intra-assay coefficients of variation of 3.58% and 4.93%, respectively. The results are shown in Table 2.
[0087] Target value (ng / mL)
Measurement times
Intra-analytical CV(%)
15.45
10
4.93
39.17
10
3.58
[0088] Table 2.
[0089] 1.2 Batch-to-batch precision analysis
[0090] Get three batches of the test kit in embodiment 1, each batch of kits all measures the quality control product liquid se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com