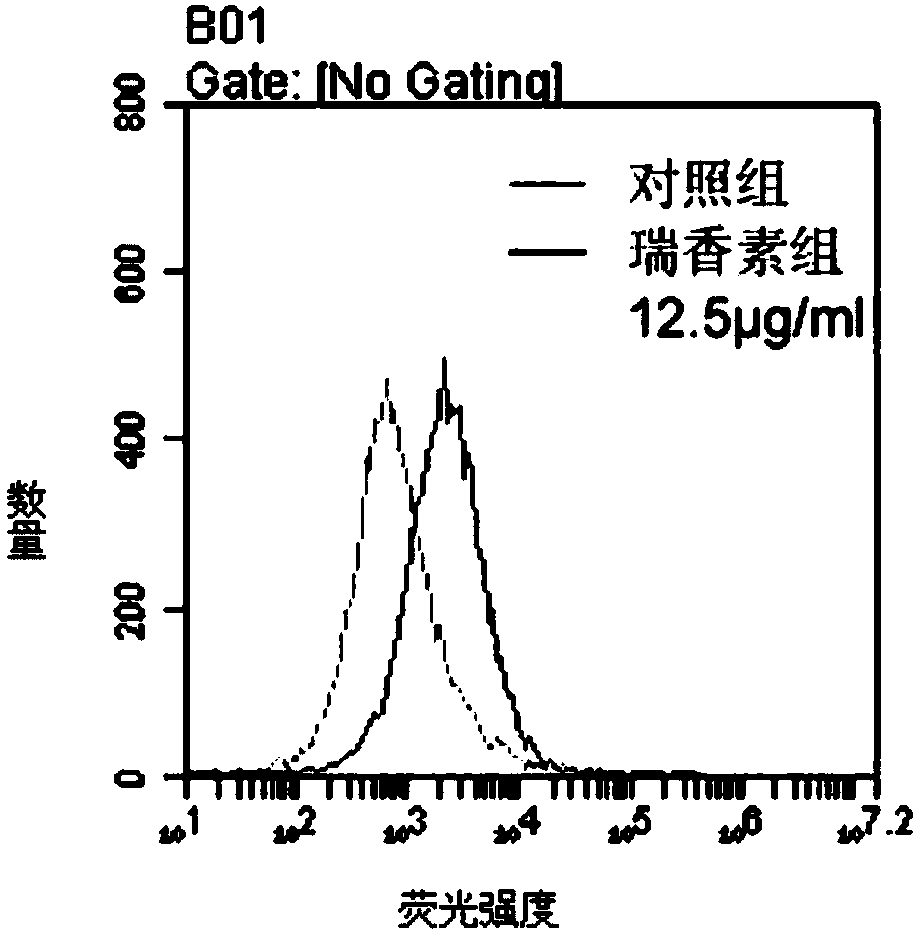
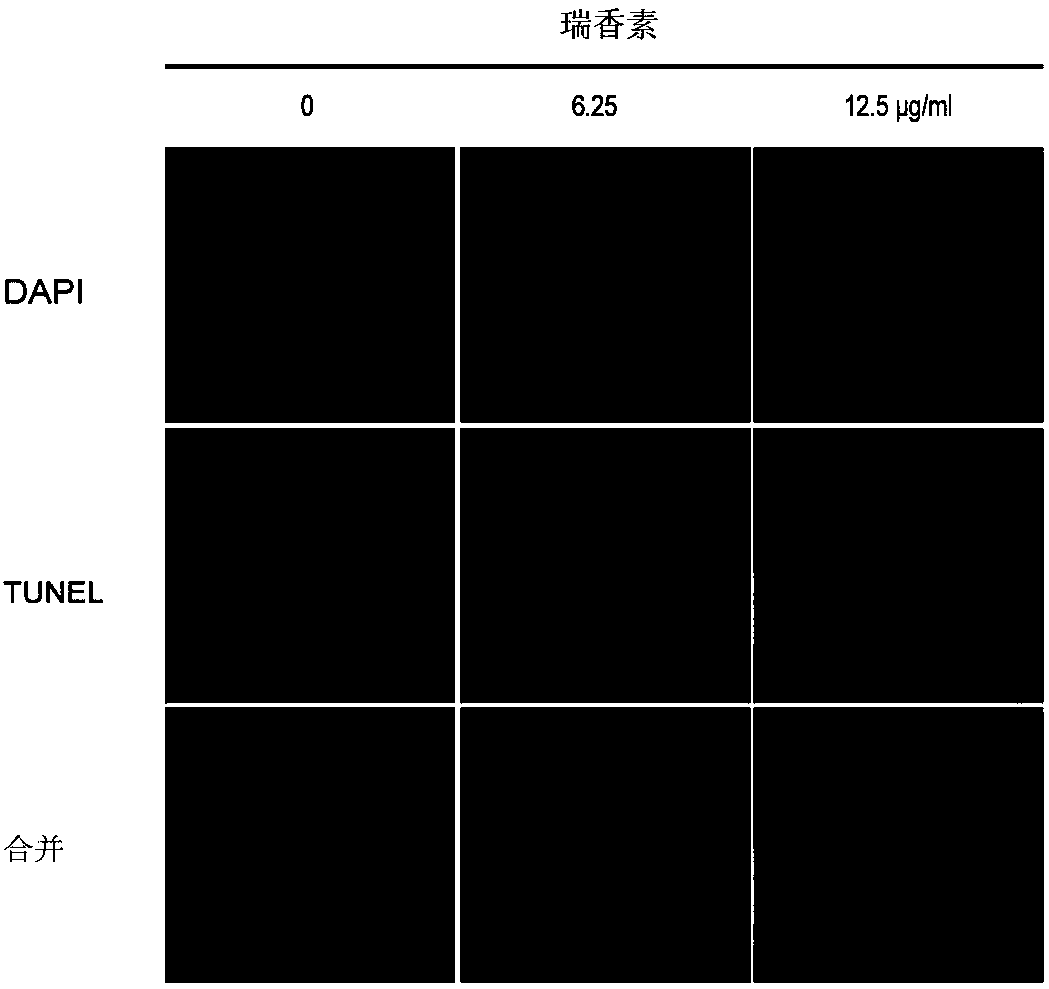
Applications of daphnetin in inhibition of Helicobacter pylori
A technology of Helicobacter pylori and daphnetin, applied in the field of daphnetin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Amoxicillin critical plate: Dissolve Mueller Hinton Agar medium in double distilled water, 121°C, 15min. When it cools down to 55°C, add 5% sheep blood, mix well, then take 13.5ml and pour it into the mixture containing 5mg / ml, 10mg / ml, 20mg / ml amoxicillin China Institute for Food and Drug Control, production batch number 130409- 200208), dry it for later use. Example 1 The acquisition of multidrug-resistant Helicobacter pylori strains
[0032] 1. Culture and subculture of Helicobacter pylori
[0033] The collected Helicobacter pylori strains were cultured in Karmali blood plates, and the culture conditions were 10% CO 2 , 5%O 2 and 85%N 2 , and cultured at 37°C for 3 days. When Helicobacter pylori grows, take it out for passage, take out the Helicobacter pylori to be passaged from the micro-aerobic tank, put it into the ultra-clean workbench, scrape enough Helicobacter pylori colonies into the normal saline with the inoculation loop, and adjust the concentration t...
Embodiment 2
[0037] The determination of embodiment 2 clinical strain drug resistance phenotype
[0038] 1. Critical value method:
[0039] (1) Inoculation: Use an inoculation loop to scrape Hp grown in the resistant plate into normal saline, and dilute to McF2.0-4.0 (about 1×10 8 CFU / ml). Use a pipette gun to add the diluted bacterial solution (2.5 μL / spot) into critical plates containing different types and concentrations of antibiotics.
[0040] The types of antibiotics are specifically metronidazole MTZ, clarithromycin CLA, levofloxacin LEV, amoxicillin AMX, and tetracycline TE.
[0041] (2) Cultivation: Put the plate into the culture tank and put it into the gas bag so that the gas condition is 10% CO 2 , 5%O 2 and 85%N 2 , and cultivated at 37°C for 72h.
[0042] (3) Result reading:
[0043] If bacteria grow in the critical plate, it is recorded as the drug-resistant bacteria of the corresponding antibiotic.
[0044] If the bacteria grow below the critical value but the critica...
Embodiment 3
[0057] Embodiment 3 drug susceptibility test
[0058] 1. Paper method
[0059] (1) Coating: Use an inoculation loop to scrape Hp grown in the resistant plate into normal saline, and dilute to McF2.0-4.0 (about 1×10 8 CFU / ml). Use a pipette gun to spread 150 μL of the diluted bacterial solution on an MH blood plate to dry, and then place a drug-sensitive paper sheet (oxioid, production batch number) containing 100 μg daphnetin (Dalian Meilun Biotechnology Co., Ltd., production batch number A1228AS) 1829617) pasted in.
[0060] (2) Cultivation: Put the plate into the culture tank and put it into the gas bag so that the gas condition is 10% CO 2 , 5%O 2 and 85%N 2 , and cultivated at 37°C for 72h.
[0061] (3) Result reading: the diameter of the inhibition zone was measured by a vernier caliper.
[0062] 2. Plate two-fold dilution method
[0063] (1) Inoculation: Use an inoculation loop to scrape Hp grown in the resistant plate into normal saline, and dilute to McF 2.0-4....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com