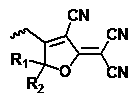
A kind of compound with d-π-a structure and its preparation method and application
A technology of compound and synthesis method, which is applied in the field of preparation of organic second-order nonlinear chromophores, can solve the problems that electronic bridges have not been reported to be used, and achieve the effects of convenient device process, high product yield, and widespread production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] An organic second-order nonlinear chromophore with D-π-A structure based on indacene derivatives was synthesized as shown below:
[0084]
[0085] The synthetic route is as follows:
[0086]
[0087] 1) Synthesis of compound 2
[0088] 44 mg of phosphine salt (0.092 mmol) and 100 mg of indacene derivative (compound 1, 0.093 mmol) were dissolved in 1,2-dichloroethane solvent, and 10 mg of sodium hydride (0.42 mmol) was slowly added at 0°C. Stir at room temperature for 24 hours, pour the reaction product into water, separate the layers, extract the aqueous phase with dichloromethane, combine the organic phases, dry the organic phase with anhydrous magnesium sulfate, filter, and remove the dichloromethane by rotary evaporation to obtain a crude product. Column chromatography (stationary phase is 200-300 mesh silica gel, mobile phase is a mixture of petroleum ether and ethyl acetate, petroleum ether: ethyl acetate = 3:1) to obtain 70 mg of yellow powder with a yield ...
Embodiment 2
[0096] An organic second-order nonlinear chromophore with D-π-A structure based on indacene derivatives was synthesized as shown below:
[0097]
[0098] The synthetic route is as follows:
[0099] 1) Synthesis of compound 2-2
[0100] 63 mg of phosphine salt (0.103 mmol) and 100 mg of indacene derivative (compound 1-2, 0.104 mmol) were dissolved in 1,2-dichloroethane solvent, and 11 mg of sodium hydride (0.46 mmol) was slowly added at 0°C. Stir at room temperature for 24 hours, pour the reaction product into water, separate the layers, extract the aqueous phase with dichloromethane, combine the organic phases, dry the organic phase with anhydrous magnesium sulfate, filter, and remove the dichloromethane by rotary evaporation to obtain a crude product. Column chromatography (stationary phase is 200~300 mesh silica gel, mobile phase is a mixture of petroleum ether and ethyl acetate, petroleum ether: ethyl acetate = 3:1), and 85 mg of powdery compound 3 is obtained with a ...
Embodiment 3
[0106] An organic second-order nonlinear chromophore with D-π-A structure based on indacene derivatives was synthesized as shown below:
[0107]
[0108] The synthetic route is as follows:
[0109]
[0110] 1) Synthesis of compound 2-3
[0111] 55 mg of phosphine salt (0.083 mmol) and 100 mg of indacene derivative (compound 1-3, 0.084 mmol) were dissolved in 1,2-dichloroethane solvent, and 9 mg of sodium hydride (0.375 mmol) was slowly added at 0°C. Stir at room temperature for 24 hours, pour the reaction product into water, separate the layers, extract the aqueous phase with dichloromethane, combine the organic phases, dry the organic phase with anhydrous magnesium sulfate, filter, and remove the dichloromethane by rotary evaporation to obtain a crude product. Column chromatography (stationary phase is 200-300 mesh silica gel, mobile phase is a mixture of petroleum ether and ethyl acetate, petroleum ether: ethyl acetate = 3:1) to obtain 76 mg of yellow powder with a yiel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com