A kind of detection method of cinnarizine related substance
A technology of related substances and detection methods, which is applied in the detection field of cinnarizine-related substances, can solve the problems of unrecorded cinnarizine, etc., and achieve the effect of accurate and reliable results, simple method and high separation degree
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] instrument:
[0035] High performance liquid chromatography, Waters, 2695-2487; electronic balance, SQP; pH meter, Shanghai Jingke PHS-3C.
[0036] Reagent:
[0037] Acetonitrile (chromatographically pure), Anhui Shilian Special Solvent Co., Ltd.; methanol (chromatographically pure), Anhui Shilian Special Solvent Co., Ltd.; glacial acetic acid (analytical pure), Tianjin Yongda Chemical Reagent Co., Ltd.; ammonium acetate (analytical pure) , Tianjin Yongda Chemical Reagent Co., Ltd.
[0038] Chromatographic conditions:
[0039] Chromatographic column: Venusil XBP C18 (4.0×100mm); flow rate, 1.0ml / min; detection wavelength, 230nm; sample concentration, 2.5mg / ml; injection volume, 10μl.
[0040] mobile phase:
[0041] Phase A: 10g / L ammonium acetate solution (pH5.0); Phase B: 0.02% glacial acetic acid in acetonitrile solution.
[0042] Elution was performed according to the following gradient program:
[0043] .
[0044] Solution preparation:
[0045] Preparation...
Embodiment 2
[0080] Chromatographic conditions:
[0081] Chromatographic column: Venusil XBP C18 (4.0×100mm); flow rate, 0.8ml / min; detection wavelength, 230nm; sample concentration, 2.5mg / ml; injection volume, 10μl.
[0082] Others are all the same as in Example 1.
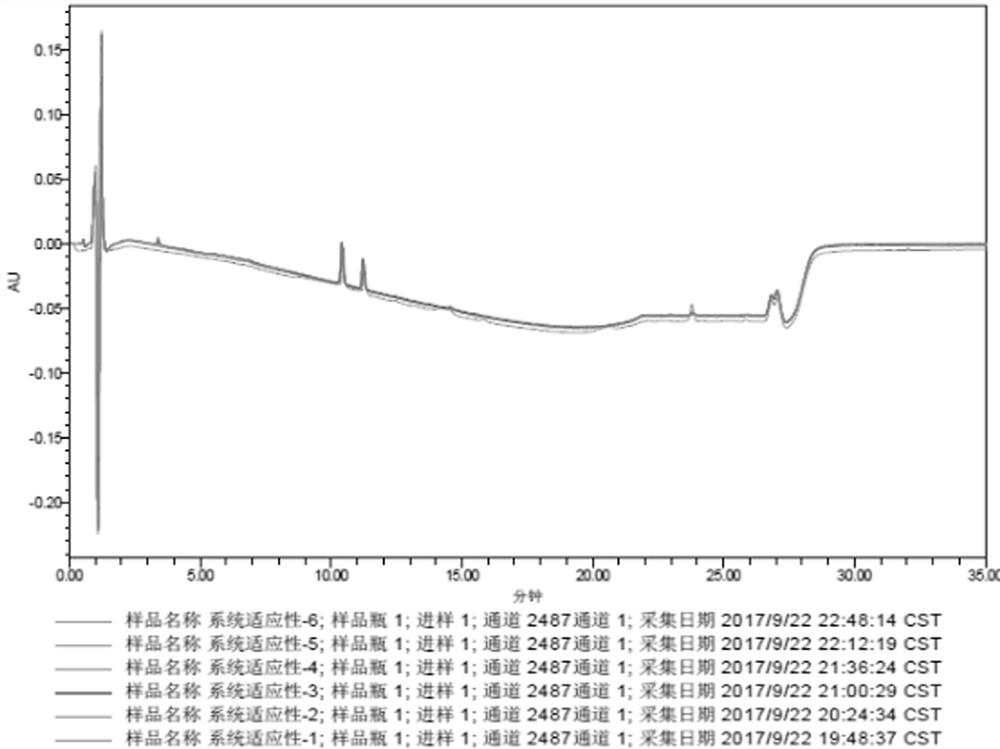
[0083] Precisely measure 10 μL each of the blank solvent, mixed reference solution, test solution and control solution, inject them into the liquid chromatograph, and record the chromatograms.
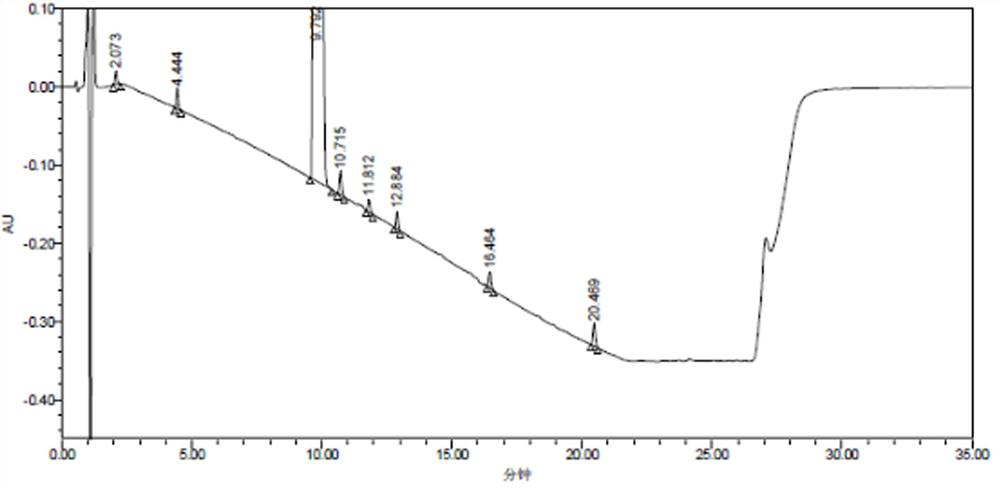
[0084] The detection results of related substances in the mixed reference solution are shown in Table 7, and the obtained HPLC collection of samples is shown in Table 7. Figure 4 ,pass Figure 4 It can be seen that all impurity peaks have been completely separated, and eight impurity peaks with retention times (t) of 2.619, 5.344, 10.942, 11.932, 13.208, 14.069, 17.953, and 22.073min were separated, especially those with the main The component peak (t=10.942min) is completely separated from the impurity peak impurity B (t=11.932mi...
Embodiment 3
[0094] Chromatographic conditions:
[0095] Chromatographic column: Venusil XBP C18 (4.0×100mm); flow rate, 1.2ml / min; detection wavelength, 230nm; sample concentration, 2.5mg / ml; injection volume, 10μl.
[0096] Others are all the same as in Example 1.
[0097] Precisely measure 10 μL each of the blank solvent, mixed reference solution, test solution and control solution, inject them into the liquid chromatograph, and record the chromatograms.
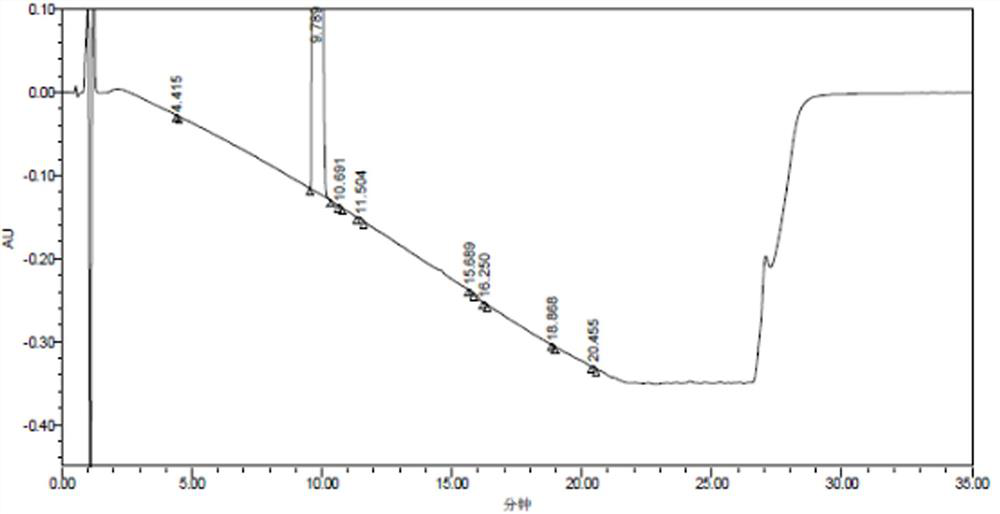
[0098] The detection results of related substances in the mixed reference solution are shown in Table 10, and the resulting HPLC spectrum is shown in Table 10. Figure 7 ,pass Figure 7 It can be seen that all impurity peaks have been completely separated, and eight impurity peaks with retention times (t) of 1.767, 3.939, 9.318, 10.013, 11.009, 12.270, 15.597, and 19.572min were separated, especially those with the main The impurity peak impurity B (t=10.013min) adjacent to the component peak (t=9.138min) has been completely separa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com