Yellow phosphorescent Cu (I) complex luminescent material and preparation method thereof
A technology of luminescent materials and complexes, applied in luminescent materials, 1/11 group organic compounds without C-metal bonds, copper organic compounds, etc., can solve the problems of short excited state lifetime, low luminous brightness, etc. Effects of radiation attenuation, increased transition probability, and ease of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
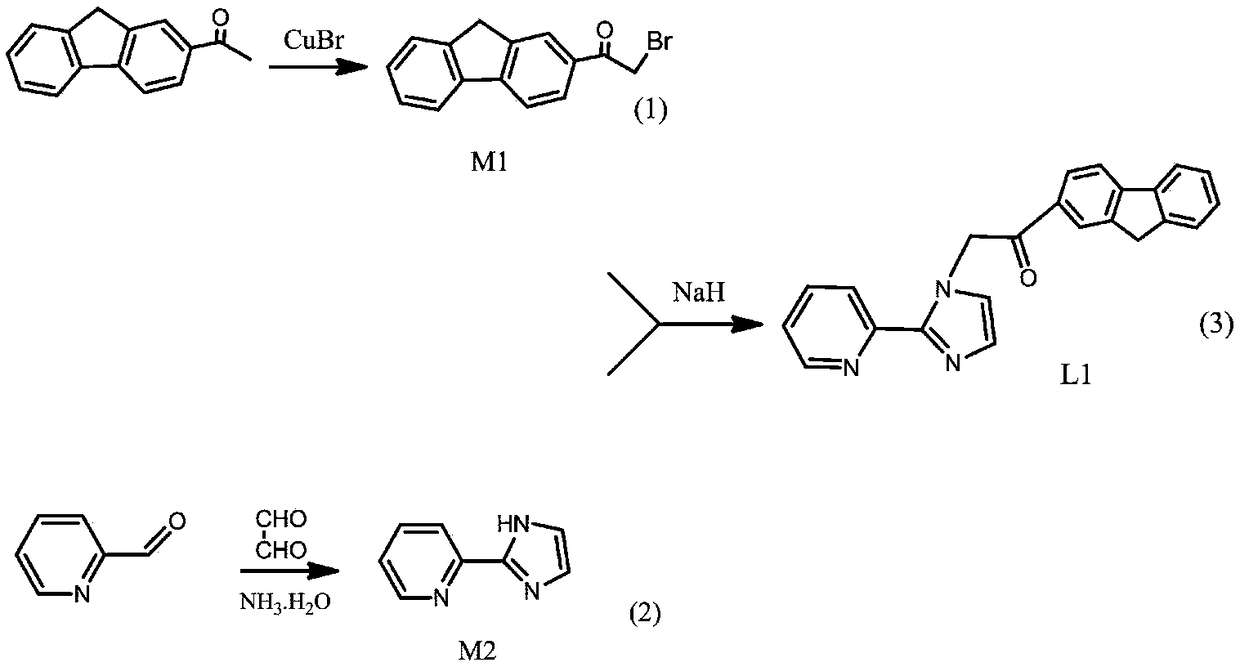
[0027] Synthesis of Ligand L1 (2-OFPI).
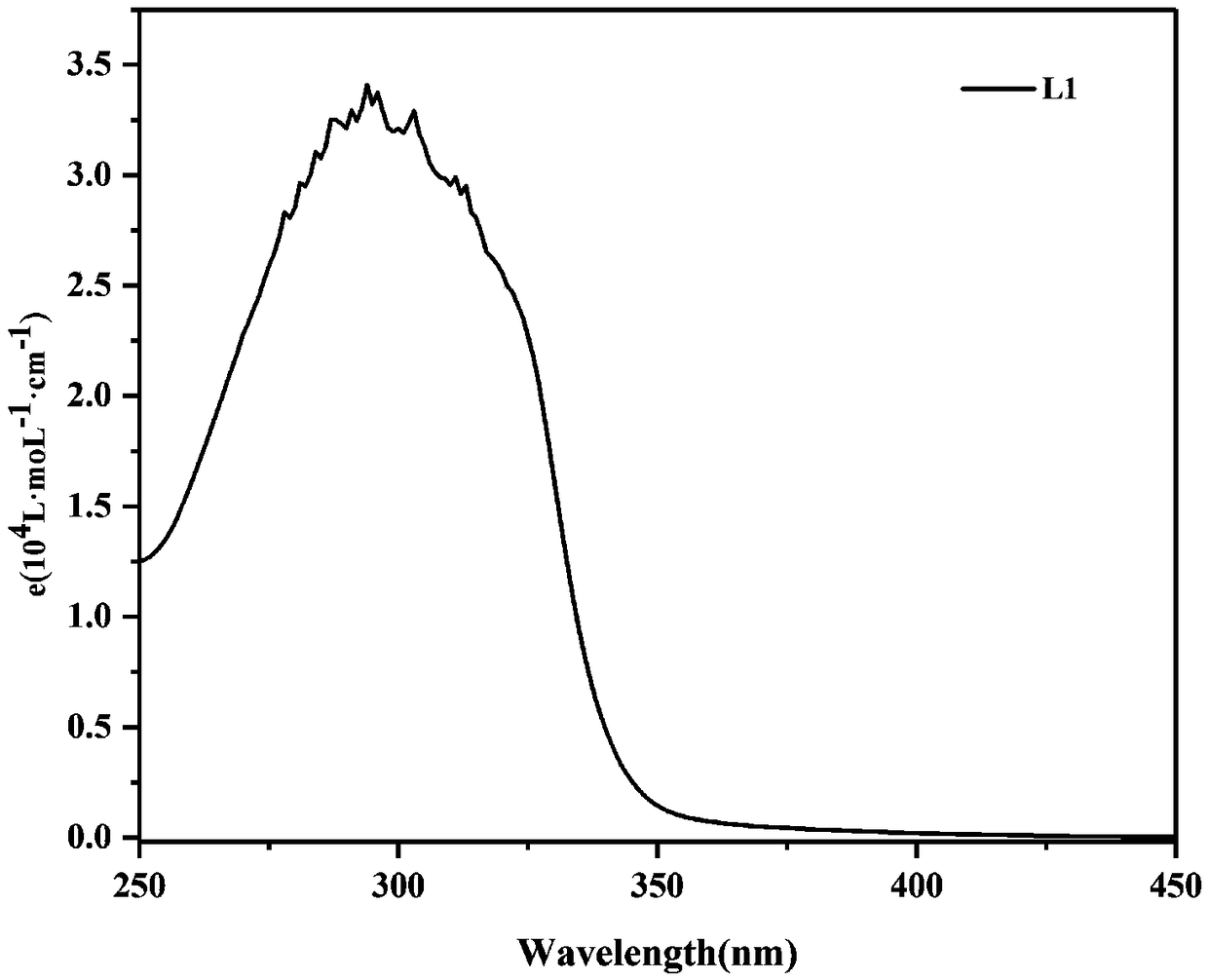
[0028] As attached to the manual figure 1 The synthetic route is shown as:
[0029] (1) Synthetic ligand L1 (2-OFPI) precursor M1 (2-bromo-1-(9H-fluoren-2-yl) ethyl ketone), the structural formula is: (III)
[0030]
[0031] Add copper bromide (2.68g, 12mmol) and 25mL of ethyl acetate to a 50mL round bottom flask, transfer the above mixture to an oil bath, stir and reflux, and dissolve 2-acetylfluorene (1.25g, 6mmol) In 13mL of dichloromethane solution, slowly add dropwise to the above solution, and the dropwise addition is completed within 15min. After stirring and refluxing for 3.5h, filter through diatomaceous earth while it is hot, wash with ethyl acetate several times, and spin the filtrate under reduced pressure. The solvent was removed by evaporation to give a gray-green solid.
[0032] (2) Synthetic ligand L1 (2-OFPI) precursor M2 (2-(2-pyridyl) imidazole), the structural formula is: (VI)
[0033]
[0034] (3) Synthet...
Embodiment 2
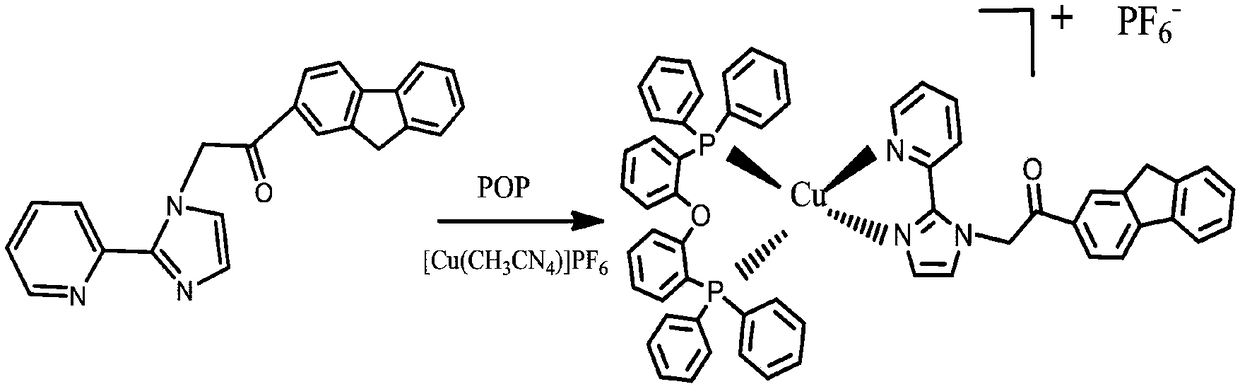
[0037] Phosphorescent complex Cu(2-OFPI)(POP)(PF 6 ) Synthesis: as attached to the instructions image 3 As shown in the synthetic route, in a 50mL round-bottomed flask, add tetraacetonitrile copper hexafluorophosphate {[Cu(CH 3 EN) 4 ](PF 6) (0.062g, 0.2mmol)}, POP (0.108g, 0.2mmol) and dichloromethane solution (10mL), stirred at room temperature under nitrogen protection for 2h, and then dissolved 0.2mmol of ligand L1 (2-OFPI) in the Inject the degassed dichloromethane solution into the above mixed solution, react at room temperature for 2 hours, filter the reacted solution through diatomaceous earth, remove the solvent by rotary evaporation of the filtrate under reduced pressure to obtain a solid powder, and distill the powder with a small amount of dichloro The methane solution was dissolved, and a small amount of acetonitrile and a large amount of ether were added. It was observed that a large amount of precipitates were produced, and the precipitates were collected by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Luminous life | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com