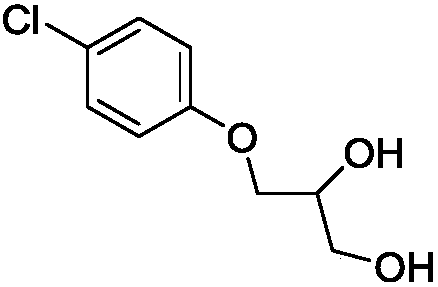
Method for green, efficient and selective synthesis of chlorphenesin
A chlorphenesin, selective technology, applied in the fields of pharmaceutical synthesis and cosmetic production, can solve the problems of coma, increased reaction steps, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
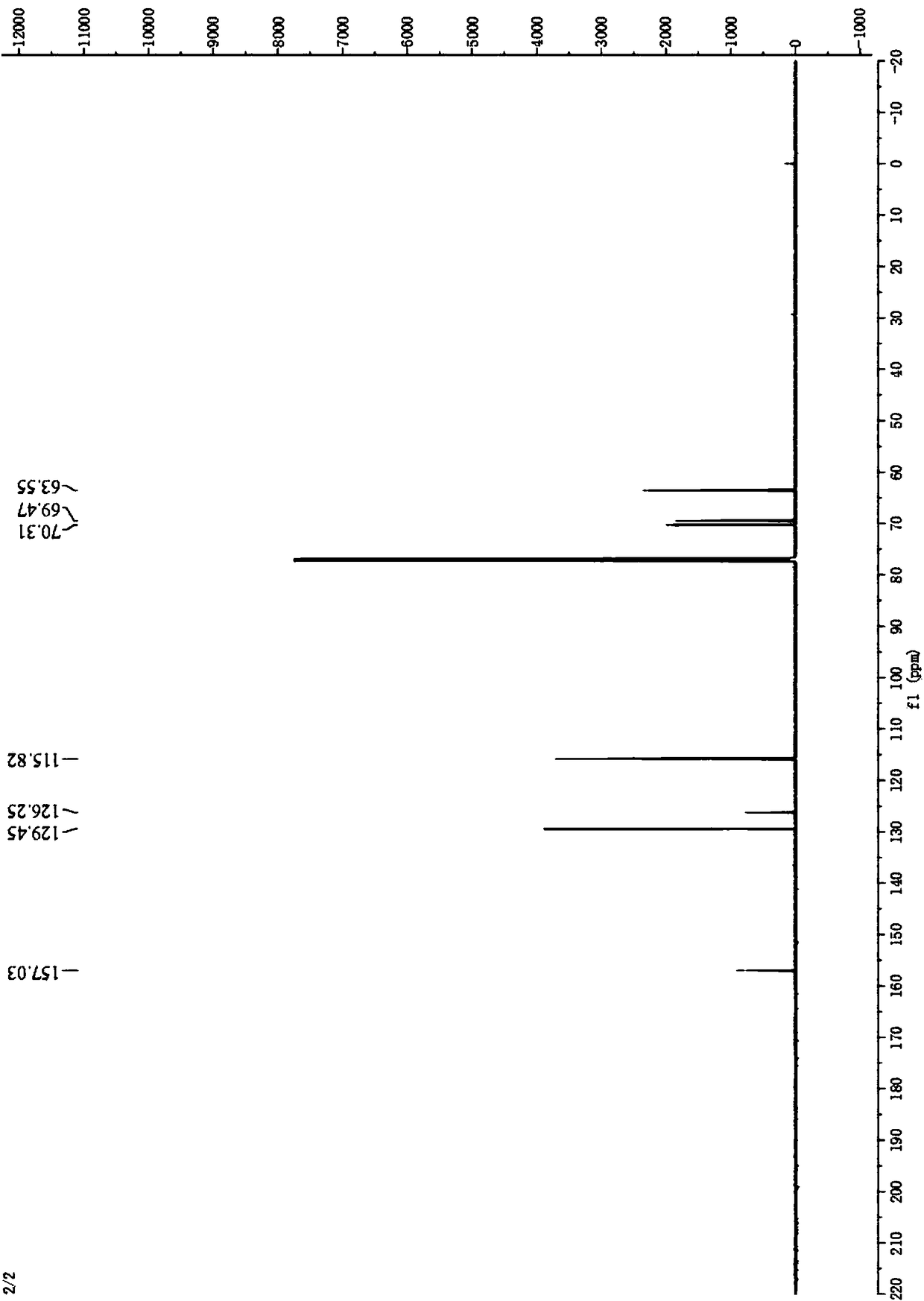
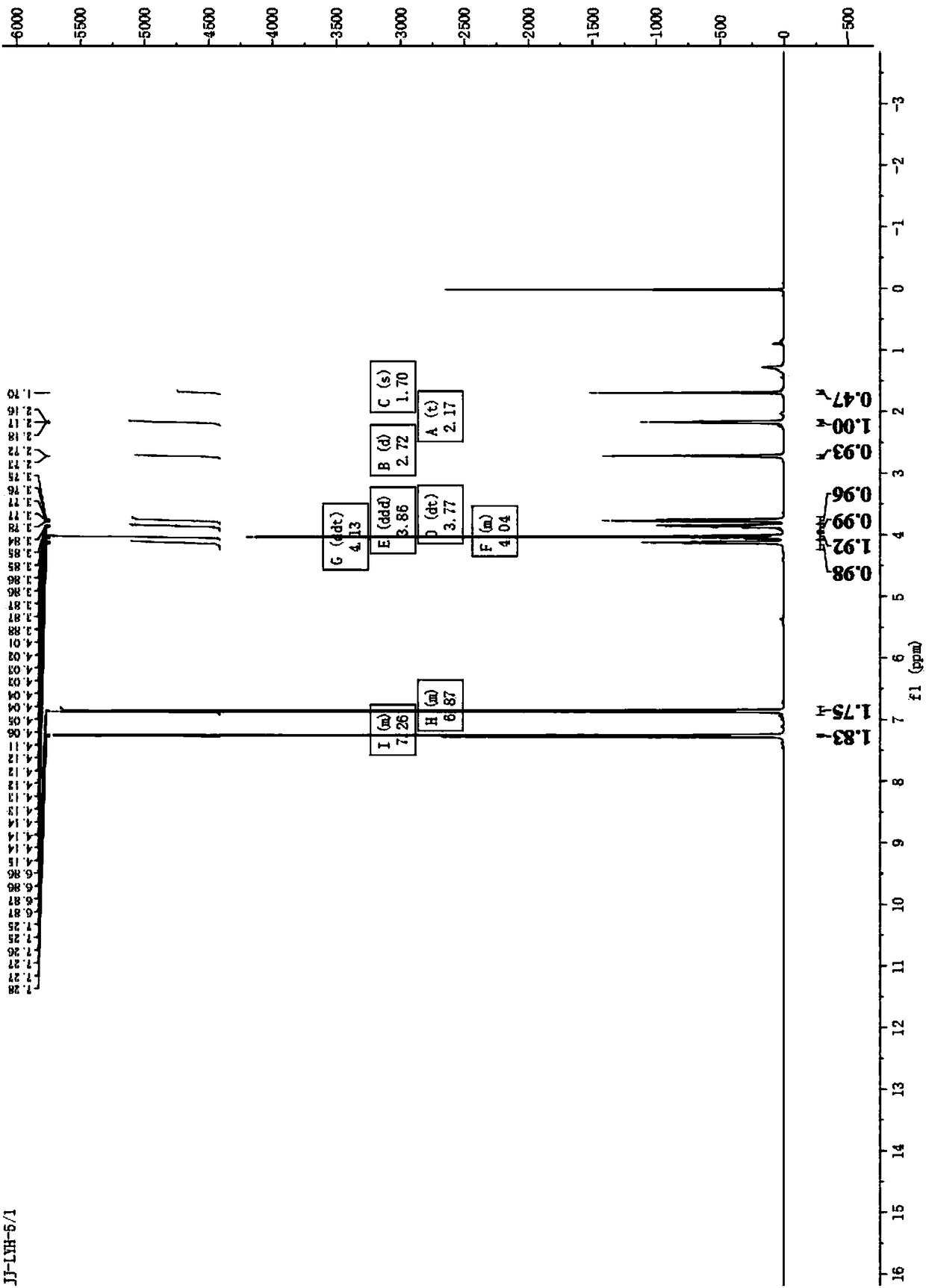
Image
Examples
Embodiment 1
[0030] A kind of method of green efficient selective synthesis of chlorphenesin, concrete operation is as follows:
[0031] In a 500ml three-necked flask, 55.25g (0.60mol) of glycerol, 25.3g (0.28mol) of dimethyl carbonate, 25.8g (0.20mol) of p-chlorophenol, and 0.8g (0.02mol) of sodium hydroxide were added successively. Wherein, the molar ratio of glycerin: diethyl carbonate: p-chlorophenol in the system is 3:1.4:1, and the molar ratio of p-chlorophenol and sodium hydroxide is 1:0.1. When feeding, keep stirring at a low speed of 30 rpm, heat to 110°C, and react for 11 hours. After the reaction, cool down to 22°C, add 552.5g of the supernatant of saturated sodium chloride solution to the above-mentioned reaction solution for washing, then add 552.5g of dichloromethane, mix well and let stand, separate layers, take the organic phase, Concentrate to obtain a yellow-white solid, which is recrystallized with dichloromethane and filtered with suction to obtain a pure white powder ...
Embodiment 2
[0033] A kind of method of green efficient selective synthesis of chlorphenesin, concrete operation is as follows:
[0034]In a 1000ml three-necked flask, 110.5g (1.20mol) of glycerin, 66g (0.56mol) of diethyl carbonate, 51.6g (0.40mol) of p-chlorophenol, and 4.04g (0.04mol) of triethylamine were added in sequence. The molar ratio of glycerin: diethyl carbonate: p-chlorophenol in the system is 3:1.4:1, and the molar ratio of p-chlorophenol and triethylamine is 1:0.1. When feeding, keep stirring at a low speed of 40 rpm, heat to 120°C, and react for 12 hours. After the reaction, lower the temperature to 24°C, add 1105g of saturated sodium chloride solution to the above-mentioned reaction solution for washing, then add 1105g of dichloromethane, mix well, let stand, separate layers, take the organic phase, concentrate to obtain a yellow-white solid , recrystallized with chloroform, and suction filtered to obtain a pure white powder product. The solid powder was vacuum-dried at 5...
Embodiment 3
[0036] A method for the selective synthesis of chlorphenesin in a green high position, the specific operations are as follows:
[0037] In a 3000ml three-necked flask, 331.5g (3.60mol) of glycerol, 245.6g (1.68mol) of dipropyl carbonate, 154.8g (1.20mol) of p-chlorophenol, and 6.72g (0.12mol) of potassium hydroxide were added successively. The molar ratio of glycerin: dipropyl carbonate: p-chlorophenol in the system is 3:1.4:1, and the molar ratio of p-chlorophenol and potassium hydroxide is 1:0.1. When feeding, keep stirring at a low speed of 50 rpm, heat to 130°C, and react for 13 hours. After the reaction, lower the temperature to 26°C, add 3315g of saturated sodium chloride solution to the above-mentioned obtained reaction solution for washing, then add 3315g of ethyl acetate, mix well, let stand, separate layers, take the organic phase, concentrate to obtain a yellow-white solid , recrystallized with ethyl acetate, and suction filtered to obtain a pure white powder produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com