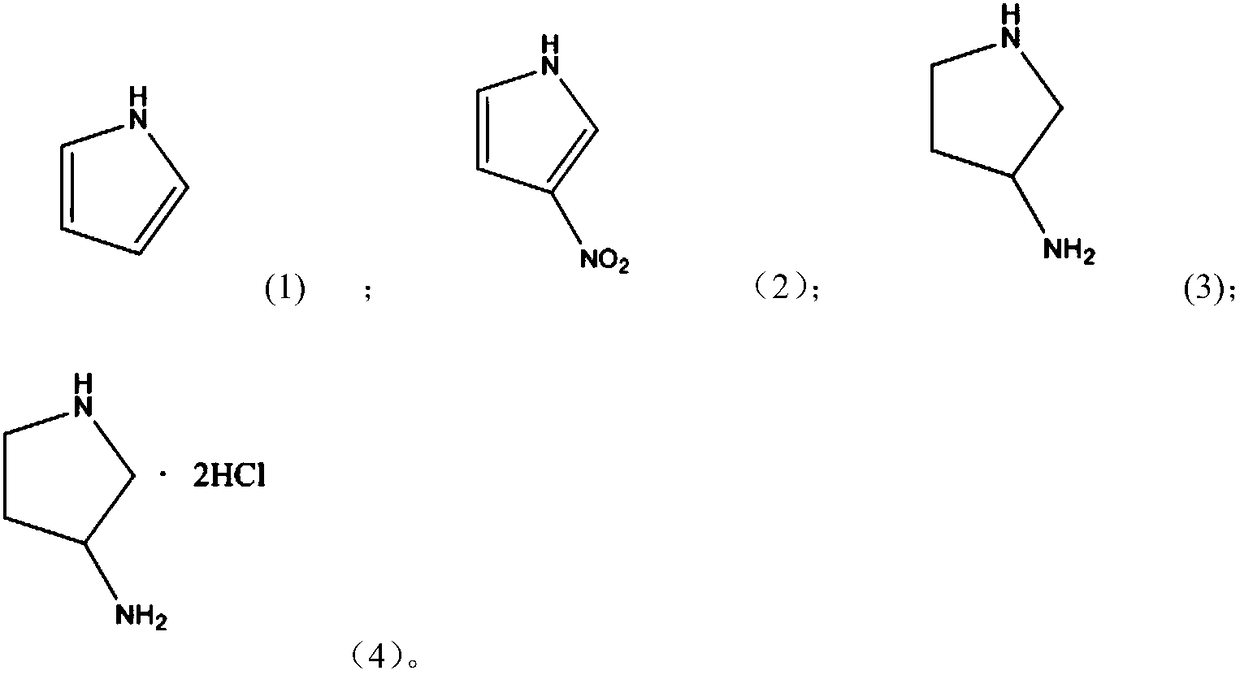
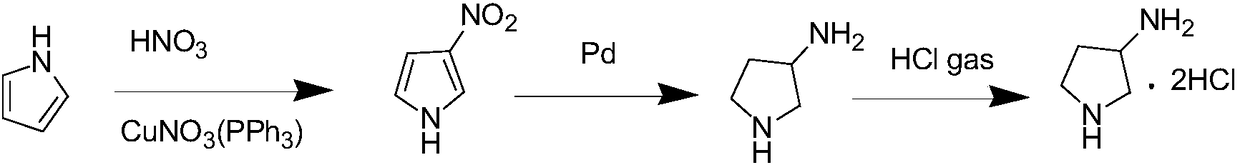
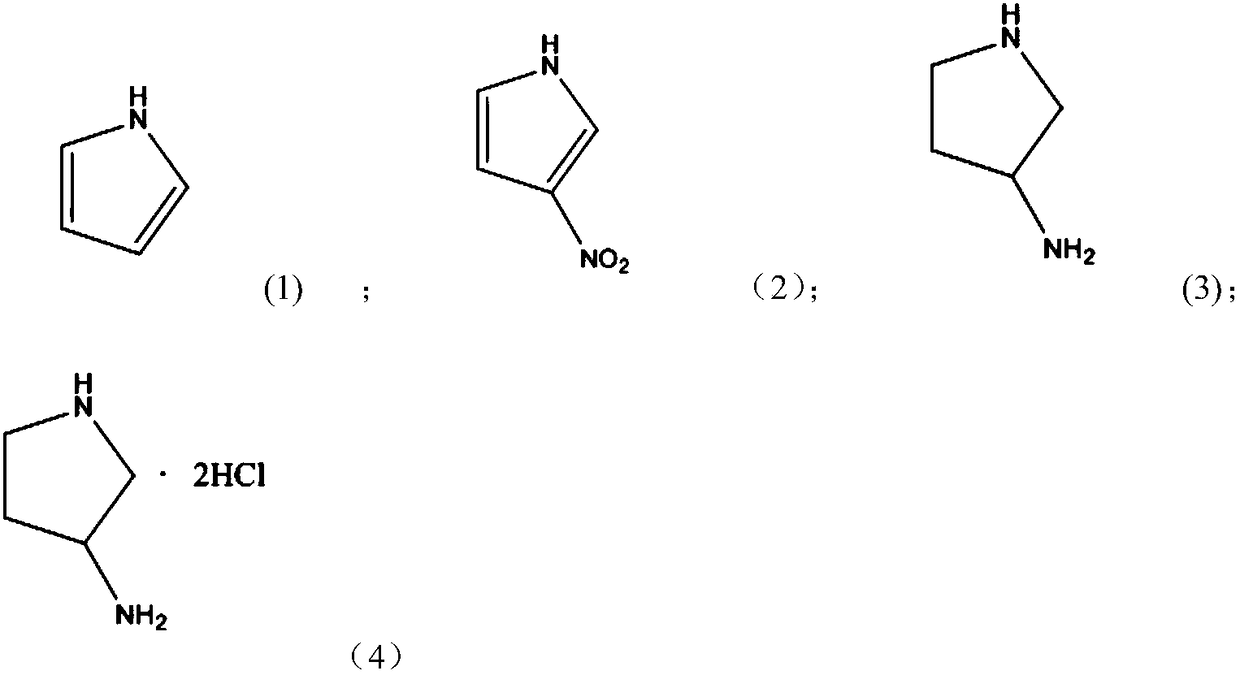
Method for preparing 3-aminopyrrolidine hydrochloride by using one-pot method
A technology of aminopyrrolidine hydrochloride and pyrrole, applied in the field of one-pot preparation of 3-aminopyrrolidine hydrochloride, can solve the problems of no industrial production, high production cost, inconvenient source, etc., and achieve complete reaction transformation, The effect of reducing pollution and reducing the cost of tail gas treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Weigh 6.70g of pyrrole, 8.8g of acetonitrile, 0.012g of bis(triphenylphosphine)copper nitrate in a 100ml flask, after cooling with ice water, slowly add 16ml of 8mol / L concentrated nitric acid dropwise, react for 1h, concentrate and add 68g of water, 100ml of ethyl acetate for extraction, and the organic layer was separated. Put the organic layer into a 250ml three-necked flask, add 8.52gPd, plug the bottle mouth with a rubber stopper, insert a glass catheter on the rubber stopper, the inlet end goes deep into the bottom of the liquid level, the gas outlet end is higher than the liquid level, and the oil bath is heated to At 80°C, hydrogen gas was slowly introduced for 1.5 hours, and the total amount of hydrogen gas introduced was 0.44 mol. Stop heating, cool to room temperature, add 26.40 g of ethyl acetate, replace the hydrogen source with HCl gas, and continue to feed HCl gas for 5 min at a flow rate of 1 L / min. After the reaction was completed, an appropr...
Embodiment 2
[0027] Example 2 Weigh 6.70g of pyrrole, 8.8g of acetonitrile, and 0.024g of bis(triphenylphosphine)copper nitrate into a 100ml flask, slowly add 18ml of 8mol / L concentrated nitric acid dropwise under ice-water bath cooling conditions, stir magnetically, and react for 1h After the reaction was completed and concentrated, 68 g of water was added, extracted with 100 ml of ethyl acetate, and the organic layer was separated. Put the organic layer into a 250ml three-necked flask, add 9.58g of Pd, plug the bottle mouth with a rubber stopper, insert a glass catheter on the rubber stopper, the inlet end goes deep into the bottom of the liquid level, the gas outlet end is higher than the liquid level, and the oil bath is heated to 85°C, slowly inject hydrogen for 1.5h. Stop heating, cool to room temperature, add 26.40 g of ethyl acetate, replace the hydrogen source with HCl gas, and continue to feed HCl gas for 6 min at a flow rate of 1 L / min. After the reaction was completed, an appr...
Embodiment 3
[0028] Example 3 Weigh 6.70g of pyrrole, 8.8g of acetonitrile, and 0.024g of bis(triphenylphosphine)copper nitrate into a 100ml flask, slowly add 20ml of 8mol / L concentrated nitric acid dropwise under ice-water cooling conditions, stir magnetically, and react for 1h After the reaction was completed and concentrated, 68 g of water was added, extracted with 100 ml of ethyl acetate, and the organic layer was separated. Put the organic layer into a 250ml three-necked flask, add 9.58g of Pd, plug the bottle mouth with a rubber stopper, insert a glass catheter on the rubber stopper, the inlet end goes deep into the bottom of the liquid level, the gas outlet end is higher than the liquid level, and the oil bath is heated to 85°C, slowly inject hydrogen for 1.5h. Stop heating, cool to room temperature, add 26.40 g of ethyl acetate, replace the hydrogen source with HCl gas, and continue to feed HCl gas for 6 min at a flow rate of 1 L / min. After the reaction was completed, an appropria...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com