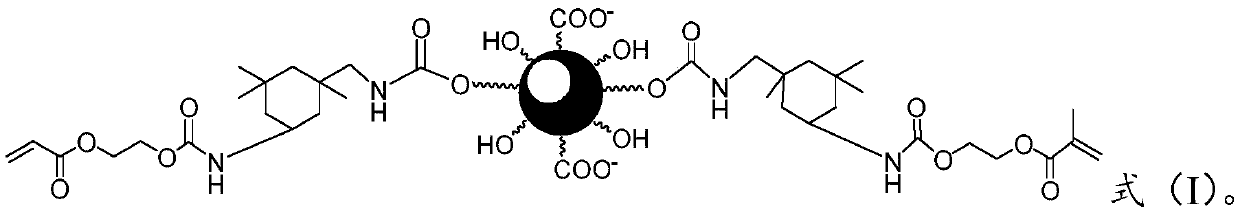
A kind of photocurable waterborne urea-isobutyraldehyde-formaldehyde resin and preparation method thereof
A technology of formaldehyde resin and isobutyraldehyde, applied in the direction of coating, etc., can solve the problems of being unfriendly to the human body and the environment, free uncured groups, water-based urea-isobutyraldehyde-formaldehyde resin is not water-soluble, etc., to achieve photocuring Fast speed, easy to automate production, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention also provides a kind of preparation method of photocurable aqueous urea-isobutyraldehyde-formaldehyde resin, comprising:
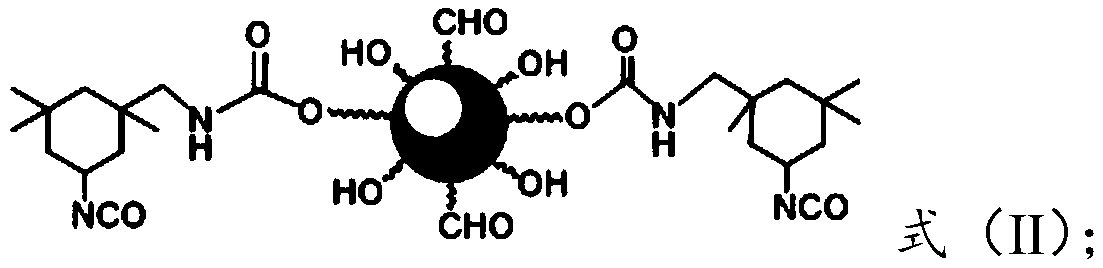
[0040] a) dissolving the urea-isobutyraldehyde-formaldehyde resin in an organic solvent and carrying out a catalytic reaction with diisocyanate under the action of a catalyst to obtain a compound of formula (II);
[0041]
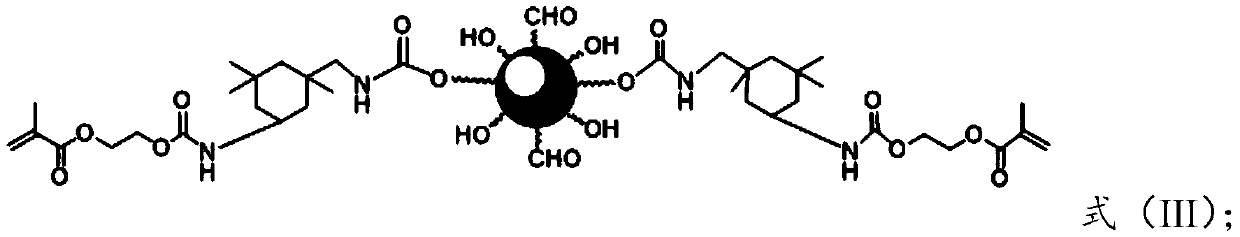
[0042] b) the compound of formula (II) is reacted with hydroxyacrylate dissolved in a polymerization inhibitor to obtain the compound of formula (III);
[0043]
[0044] c) dissolving the compound of formula (III) in an organic solvent, adding 3wt% to 10wt% hydrogen peroxide, and performing an oxidation reaction to obtain a compound of formula (IV);
[0045]
[0046] d) A salt-forming reaction is carried out between the compound of formula (IV) and a salt-forming agent to obtain a photocurable water-based urea-isobutyraldehyde-formaldehyde resin having a structure represented by formula (I).
[004...
Embodiment 1
[0087] In this example, a photocurable water-based urea-isobutyraldehyde-formaldehyde resin was prepared according to the following steps:
[0088] 1) A four-necked flask, a mechanical stirrer, a constant pressure funnel, a condenser and a thermometer are equipped on the oil bath, and 14.7 parts by mass of urea-isobutyraldehyde-formaldehyde resin and 13.5 parts by mass of butanone are added to the four-necked flask (through 4A molecular sieve drying for more than 12h), after the urea-isobutyraldehyde-formaldehyde resin is completely dissolved in butanone, weigh 0.1% of the total mass of urea-isobutyraldehyde-formaldehyde resin and butanone and add dibutyltin dilaurate (DBTDL) into a four-neck flask. Then, 8.2 parts by mass of isophorone diisocyanate (IPDI) was dropped into a four-neck bottle, and the dropwise addition was completed within 20 minutes. The temperature was raised to 80° C., and a catalytic reaction was carried out for 7 hours to obtain a compound of formula (II)....
Embodiment 2
[0093] In this example, a photocurable water-based urea-isobutyraldehyde-formaldehyde resin was prepared according to the following steps:
[0094] 1) a four-necked flask, a mechanical stirrer, a constant pressure funnel, a condenser and a thermometer are equipped on the oil bath, and 13.8 parts by mass of urea-isobutyraldehyde-formaldehyde resin and 12.7 parts by mass of methyl ethyl ketone are added to the four-necked flask (through 4A molecular sieve drying for more than 12h), after the urea-isobutyraldehyde-formaldehyde resin is completely dissolved in butanone, weigh 0.1% of the total mass of urea-isobutyraldehyde-formaldehyde resin and butanone and add dibutyltin dilaurate (DBTDL) into a four-neck flask. Then 7.7 parts by mass of isophorone diisocyanate (IPDI) was dropped into the four-neck bottle, the dropwise addition was completed within 20 minutes, the temperature was raised to 80° C., and the catalytic reaction was carried out for 7 hours to obtain the compound of f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| adhesivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com