Small-particle-size metal phosphide nanoparticle/reduced graphene composite material and preparation method thereof
A metal phosphide and nanoparticle technology, used in phosphide, chemical instruments and methods, nanotechnology, etc., can solve the problems of large catalyst particle size, easy agglomeration, and hindering the exposure of active sites.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
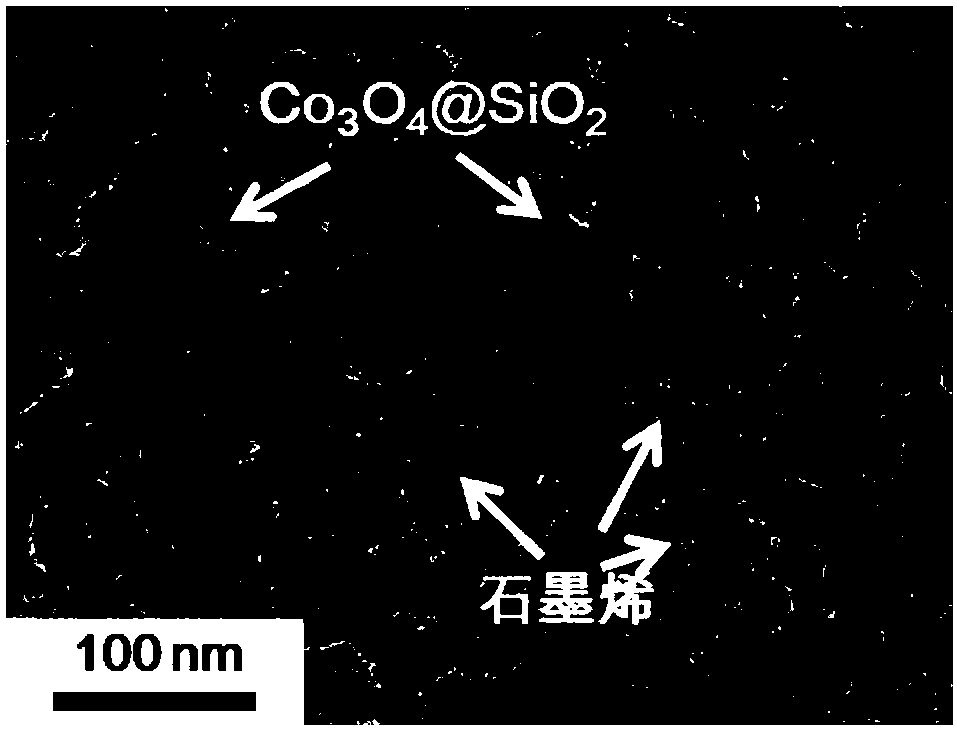
[0092] (1) preparing the composite particles of silicon dioxide coated tricobalt tetroxide, specifically comprising the following steps:
[0093] (A) 348mg cobalt nitrate tetrahydrate was dissolved in 1.2ml deionized water to obtain cobalt nitrate aqueous solution;
[0094] (B) Dissolving 10g of polyoxyethylene ether in 45ml of n-hexane, heating at 50°C until clear and transparent, to obtain a n-hexane solution of polyoxyethylene ether;
[0095] (C) Add 0.2ml of cobalt nitrate aqueous solution to 50ml of polyoxyethylene ether in n-hexane solution, and stir at 50°C for 1h;
[0096] (D) Add 2ml of ammonia water to the mixed solution obtained in step (C), and continue to stir for 1h;
[0097] (E) Add 6ml of analytically pure tetraethyl silicate to the mixed solution obtained in step (D), and continue to stir for 2h;
[0098] (F) Step (E) is separated after the stirring is completed, and then the separated product is dried at 80° C. for 12 hours;
[0099] (G) calcining the produc...
Embodiment 2
[0115] (1) preparing the composite particles of silica-coated copper oxide, specifically comprising the following steps:
[0116] (A) 250mg copper sulfate pentahydrate was dissolved in 1.2ml deionized water to obtain an aqueous solution of copper sulfate;
[0117] (B) Dissolving 10g of polyoxyethylene ether in 45ml of n-hexane, heating at 55°C until clear and transparent, to obtain a n-hexane solution of polyoxyethylene ether;
[0118] (C) Add 1ml of copper sulfate aqueous solution to 50ml of polyoxyethylene ether in n-hexane solution, and stir at 55°C for 1h;
[0119] (D) Add 1ml of ammonia water to the mixed solution obtained in step (C), and continue to stir for 1h;
[0120] (E) Add 6ml of analytically pure tetraethyl silicate to the mixed solution obtained in step (D), and continue to stir for 2h;
[0121] (F) Step (E) is separated after the stirring is completed, and then the separated product is dried at 80° C. for 12 hours;
[0122] (G) calcining the product obtained...
Embodiment 3
[0130] 1) preparing composite particles of silica-coated nickel oxide, specifically comprising the following steps:
[0131] (A) 200mg nickel chloride hexahydrate is dissolved in 1.0ml deionized water to obtain an aqueous solution of nickel chloride;
[0132] (B) Dissolve 8g of polyoxyethylene ether in 45ml of n-hexane, and heat at 52°C until clear and transparent to obtain a n-hexane solution of polyoxyethylene ether;
[0133] (C) Add 0.5ml of nickel chloride aqueous solution to 50ml of polyoxyethylene ether in n-hexane solution, and stir at 52°C for 1h;
[0134] (D) Add 0.8ml of ammonia water to the mixed solution obtained in step (C), and continue to stir for 1.2h;
[0135] (E) Add 5 ml of analytically pure tetraethyl silicate to the mixed solution obtained in step (D), and continue stirring for 2 h;
[0136] (F) Step (E) is separated after the stirring is completed, and then the separated product is dried at 80° C. for 10 h;
[0137] (G) Calcining the dried product obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com