Preparation method of anhydrous cerium chloride
A technology of samarium chloride and ammonium chloride, applied in chemical instruments and methods, inorganic chemistry, rare earth metal chlorides, etc., can solve the problems of difficulty in obtaining pure anhydrous rare earth chloride, unfavorable industrial production, and high chlorination temperature requirements , to achieve the effect of safe and effective production method, high yield of reaction product, and improvement of product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
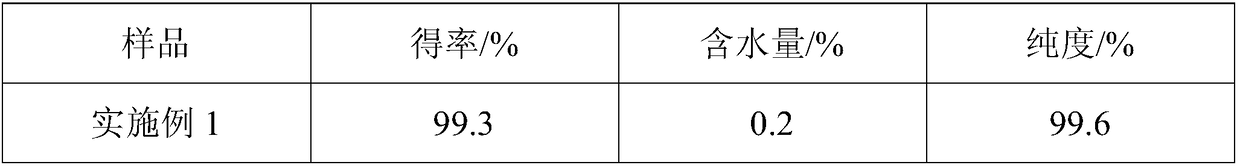
Embodiment 1
[0052] (1) Pretreatment: pulverizing samarium oxide through a 130 mesh sieve, and pulverizing ammonium chloride through a 140 mesh sieve;
[0053] (2) The ammonium chloride is tiled on the bottom of the reaction device, the tile thickness is 8cm, the samarium oxide is tiled on the ammonium chloride solid, and the mass ratio of samarium oxide to ammonium chloride is 1:2;
[0054] (3) Gradient heating, the heating rate is 16°C / min, stop heating when the temperature reaches 330°C, and react at constant temperature, the reaction time at constant temperature is 4h;
[0055] (4) 1 hour after the start of the constant temperature reaction, dry hydrogen chloride gas is introduced, and the ventilation rate is 0.6L / min;
[0056] (5) The reaction product obtained in step (4) was dried under reduced pressure for 2 hours under an inert atmosphere, the operating pressure was 3.1 kPa, and the drying temperature was 85° C. to obtain anhydrous samarium chloride.
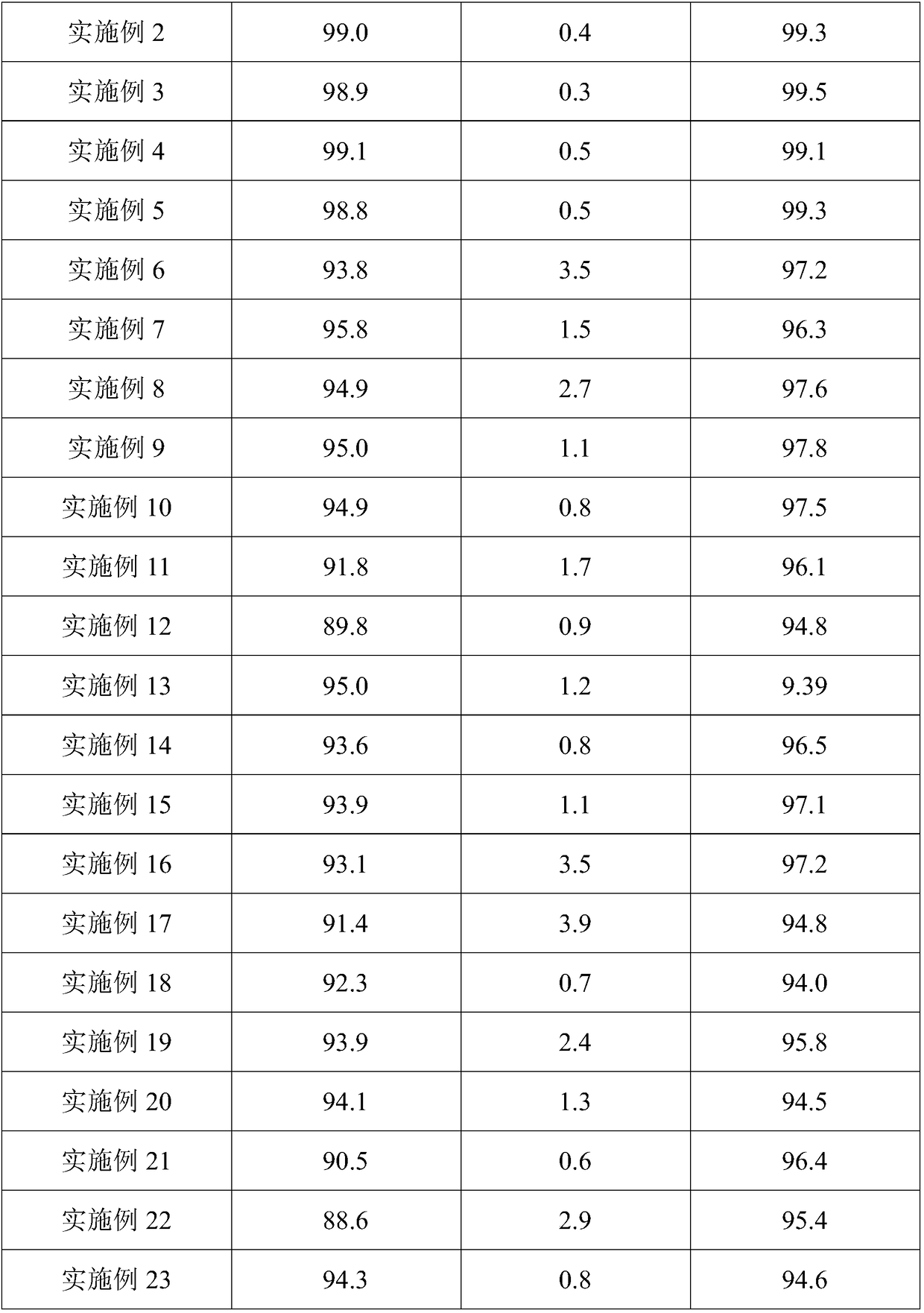
Embodiment 2
[0058] (1) Pretreatment: pulverizing samarium oxide through a 140 mesh sieve, and pulverizing ammonium chloride through a 130 mesh sieve;
[0059] (2) The ammonium chloride is tiled on the bottom of the reaction device, the tile thickness is 12cm, the samarium oxide is tiled on the ammonium chloride solid, and the mass ratio of samarium oxide to ammonium chloride is 1:1.5;
[0060] (3) Gradient temperature rise, the temperature rise rate is 12°C / min, stop heating when the temperature reaches 360°C, constant temperature reaction, constant temperature reaction time is 3h;
[0061] (4) Feed dry hydrogen chloride gas after 1.5h from the start of the constant temperature reaction, and the ventilation rate is 0.5L / min;
[0062] (5) The reaction product obtained in step (4) was dried under reduced pressure for 1 h under an inert atmosphere, the operating pressure was 4.2 kPa, and the drying temperature was 103° C. to obtain anhydrous samarium chloride.
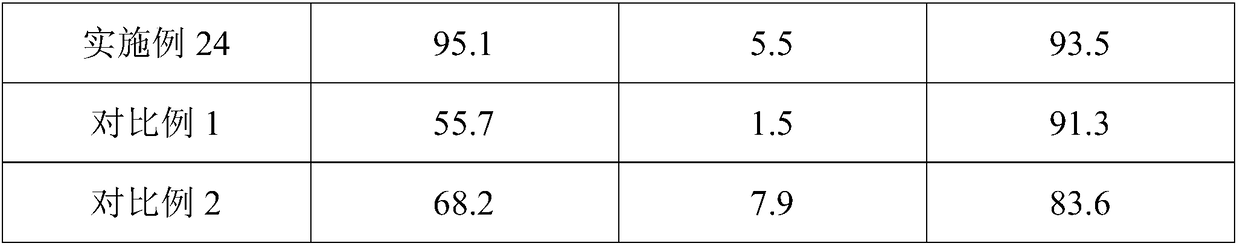
Embodiment 3
[0064] (1) Pretreatment: pulverizing samarium oxide through a 120 mesh sieve, and pulverizing ammonium chloride through a 120 mesh sieve;
[0065] (2) The ammonium chloride is tiled on the bottom of the reaction device, the tile thickness is 10cm, the samarium oxide is tiled on the ammonium chloride solid, and the mass ratio of samarium oxide to ammonium chloride is 1:2.5;
[0066] (3) Gradient heating, the heating rate is 20°C / min, stop heating when the temperature reaches 325°C, constant temperature reaction, constant temperature reaction time is 5h;
[0067] (4) 1 hour after the start of the constant temperature reaction, dry hydrogen chloride gas is introduced, and the ventilation rate is 0.8L / min;
[0068] (5) The reaction product obtained in step (4) was dried under reduced pressure for 2.5 hours under an inert atmosphere, the operating pressure was 5.3 kPa, and the drying temperature was 75° C. to obtain anhydrous samarium chloride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com