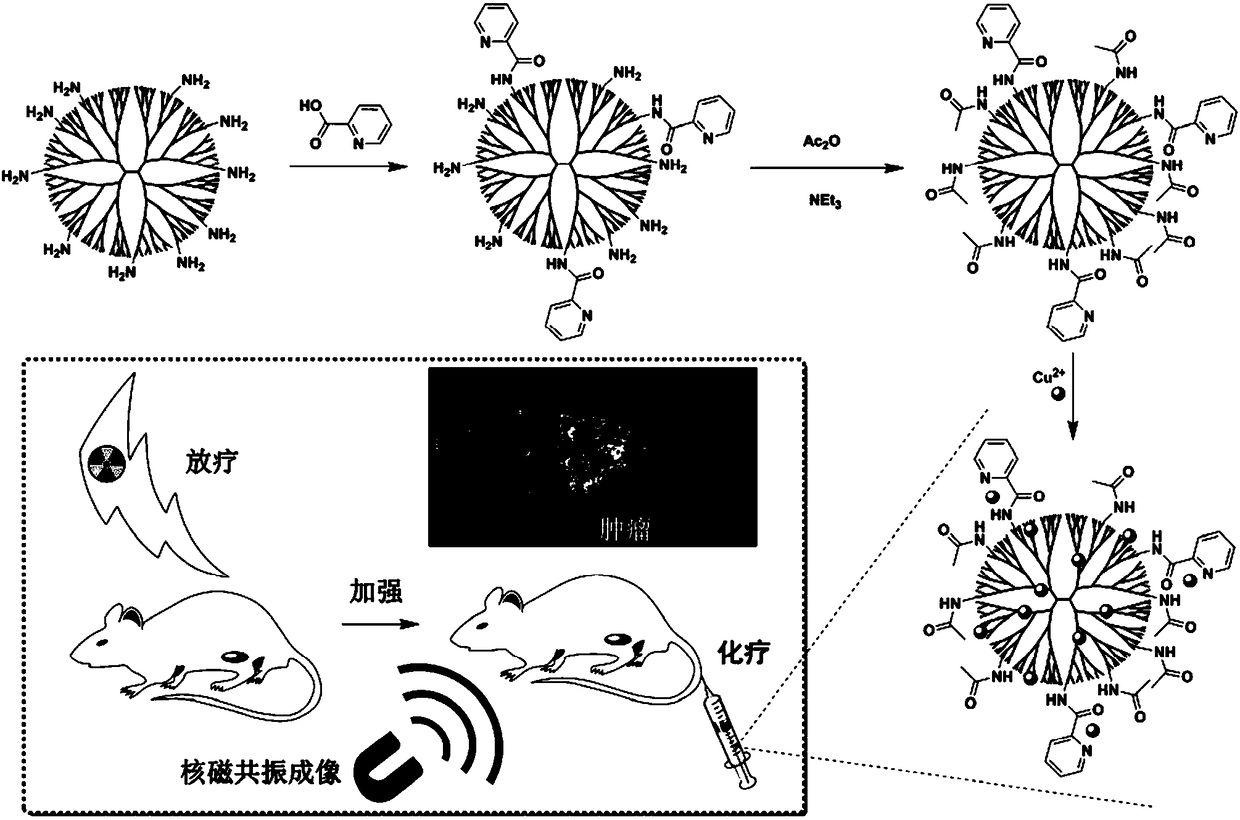
Preparation method of pyridine-modified dendrimer copper complex hybrid nanomaterial
A technology of dendrimers and nanomaterials, which is applied in the field of preparation of dendrimer copper complex hybrid nanomaterials, can solve the problems of undiscovered integration of diagnosis and treatment, and achieve the effect of promoting the integration of diagnosis and treatment, good water solubility , the effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Dissolve 2-pyridinecarboxylic acid (23.66mg) in 5mL of ultrapure water, add EDC (73.70mg) to activate for 0.5h, and then add dropwise to 5mL of the 5th generation polyamide-amine whose terminal group is an amino group Dendrimer G5.NH 2 (100mg) in an ultrapure aqueous solution (20mg / mL), stirred at room temperature for 24 hours, and then used a dialysis bag with a molecular weight cut-off of 5000 to dialyze the aqueous solution for 3 days (2L / time, 3 times / day), and finally freeze-dried to obtain pyridine-modified Dendrimer G5.NH 2 -Pyr.
[0041] (2) Change G5.NH 2 -Pyr (112.12mg) was dissolved in 5mL ultrapure water, added triethylamine (38.90mg) and mixed evenly, added dropwise acetic anhydride solution (39.25mg), stirred at room temperature for 24 hours, and then used a dialysis bag with a molecular weight cut-off of 5000 to The aqueous solution was dialyzed for 3 days (2L / time, 3 times / day), and finally freeze-dried to obtain the acetylated dendrimer-pyridine ...
Embodiment 2
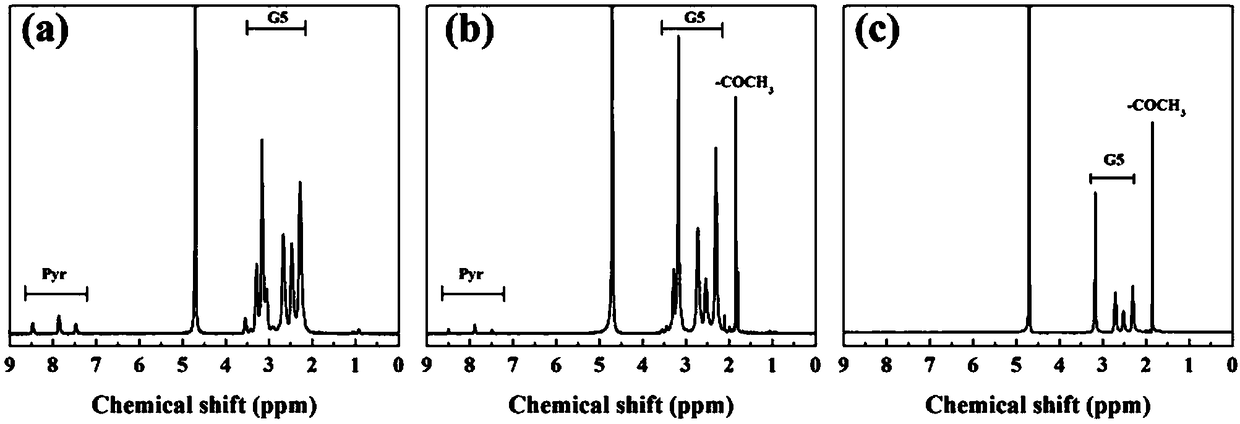
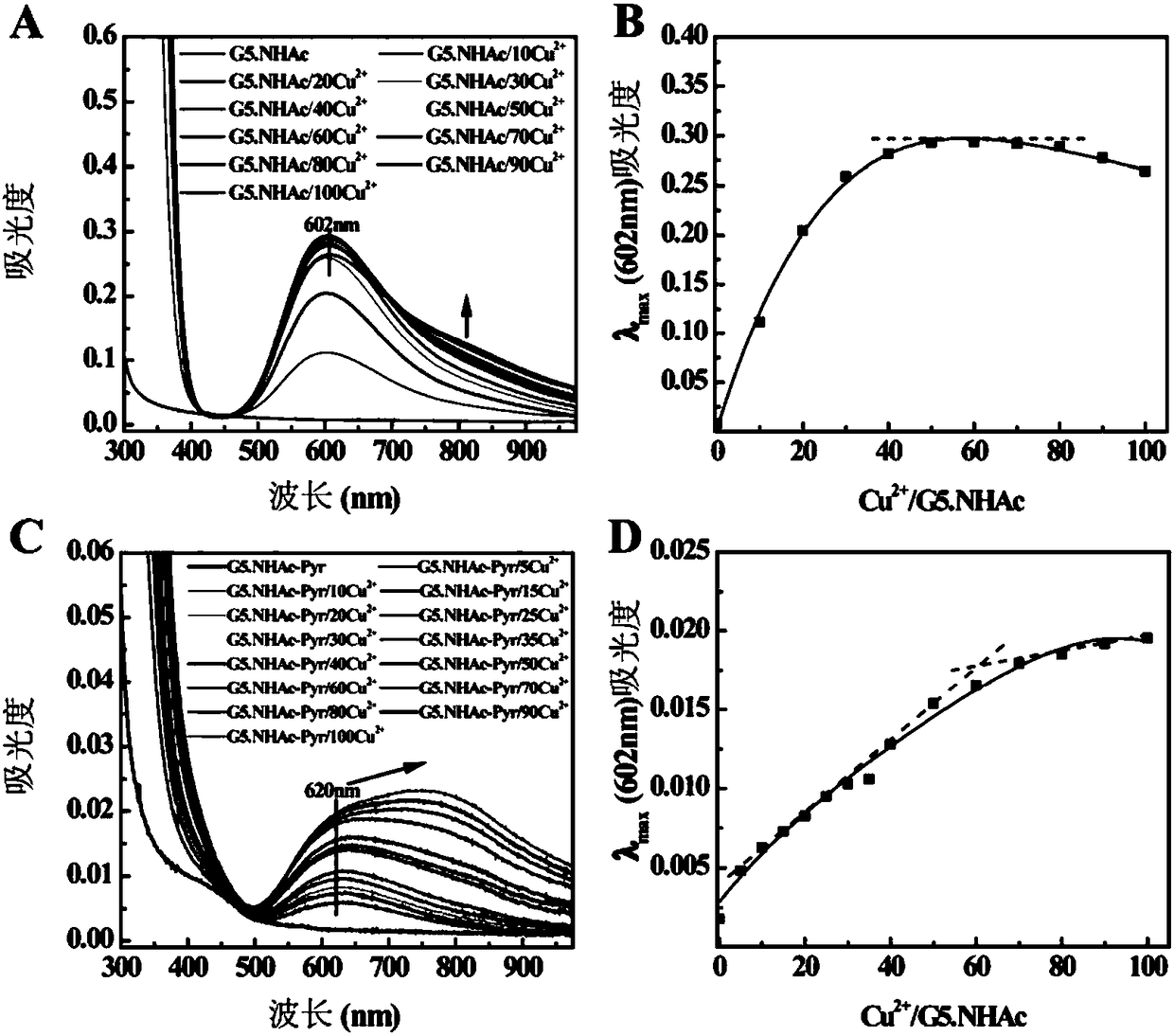
[0049] Get the G5.NH prepared in Example 1 2 -Pyr and G5.NHAc-Pyr and G5.NHAc prepared in Comparative Example 1 were dissolved in deuterated water, and Bruker 400MHz NMR was used for hydrogen spectrum testing. Depend on figure 2 a It can be seen that in G5.NH 2 -In the hydrogen nuclear magnetic resonance spectrum of Pyr, 7.5, 8 and 8.5ppm are the characteristic proton peaks on the pyridine ring, and 2.0-3.6ppm is the dendrimer G5.NH 2 The methylene proton peaks, according to the ratio of their integral areas, calculate each G5.NH 2 29.7 Pyr molecules are linked above (the molar ratio of its feed is G5.NH 2 :Pyr=1:50). Prepared G5.NH 2 After -Pyr is through acetylation reaction, the methyl proton peak of acetyl appears between 1.9ppm ( figure 2 b), indicating that the dendrimer G5.NH 2 The remaining amino groups on the surface have been acetylated. According to the ratio of their integral areas, each G5.NH after acetylation is calculated 2 -80 acetyl groups on Pyr. D...
Embodiment 3
[0054] KB cells in the logarithmic growth phase were collected, seeded on a 96-well cell culture plate at a density of 10,000 cells per well, and placed in 5% CO 2 , and incubated overnight at 37°C. After discarding the medium, replace 90 μL serum-free medium per well, and add 10 μL containing different concentrations of materials (final material concentrations are 0.01, 0.1, 1, 2, 5, 10 mM) or saline (control group). Then place the cell culture plate in 5% CO 2 , and continue to incubate for 24 hours at 37°C. Then the original culture medium was discarded, and 100 μL of fresh culture medium solution containing 10% CCK-8 was added. After continuing to cultivate for 3 hours, it was placed in a multifunctional microplate reader to test the absorbance value at a test wavelength of 450 nm. The results were as follows: Figure 5As shown in A. Compared with the normal saline control group, G5.NHAc has no obvious cytotoxicity to KB cells within the tested concentration range, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com