Preparation method of peroxide substituted pyrrole/furan indoline
An indoline and pyrrole technology, applied in the field of fine chemical catalysis synthesis, can solve the problems of unstable reaction conditions, low peroxy bond energy, sensitivity, etc., and achieve the effects of strong operability, wide sources and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
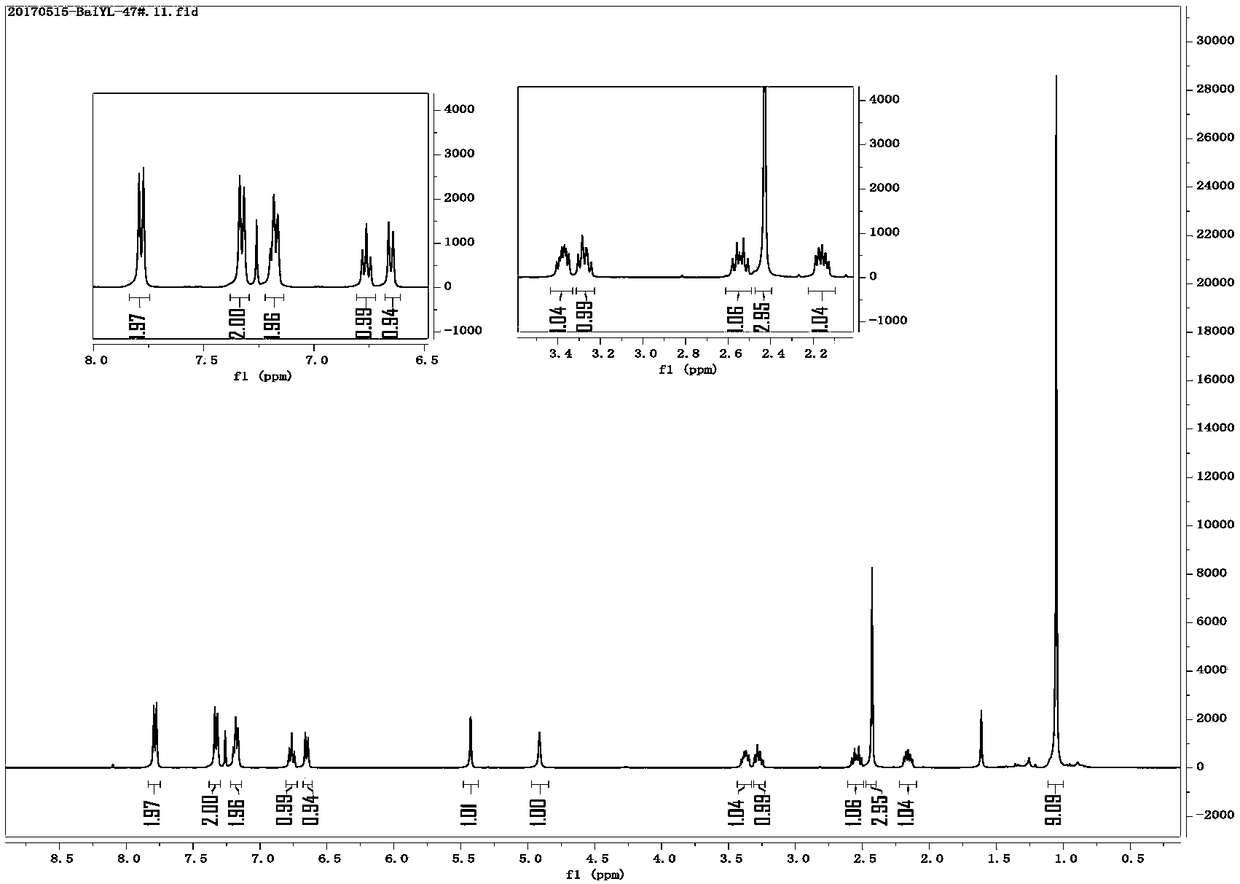
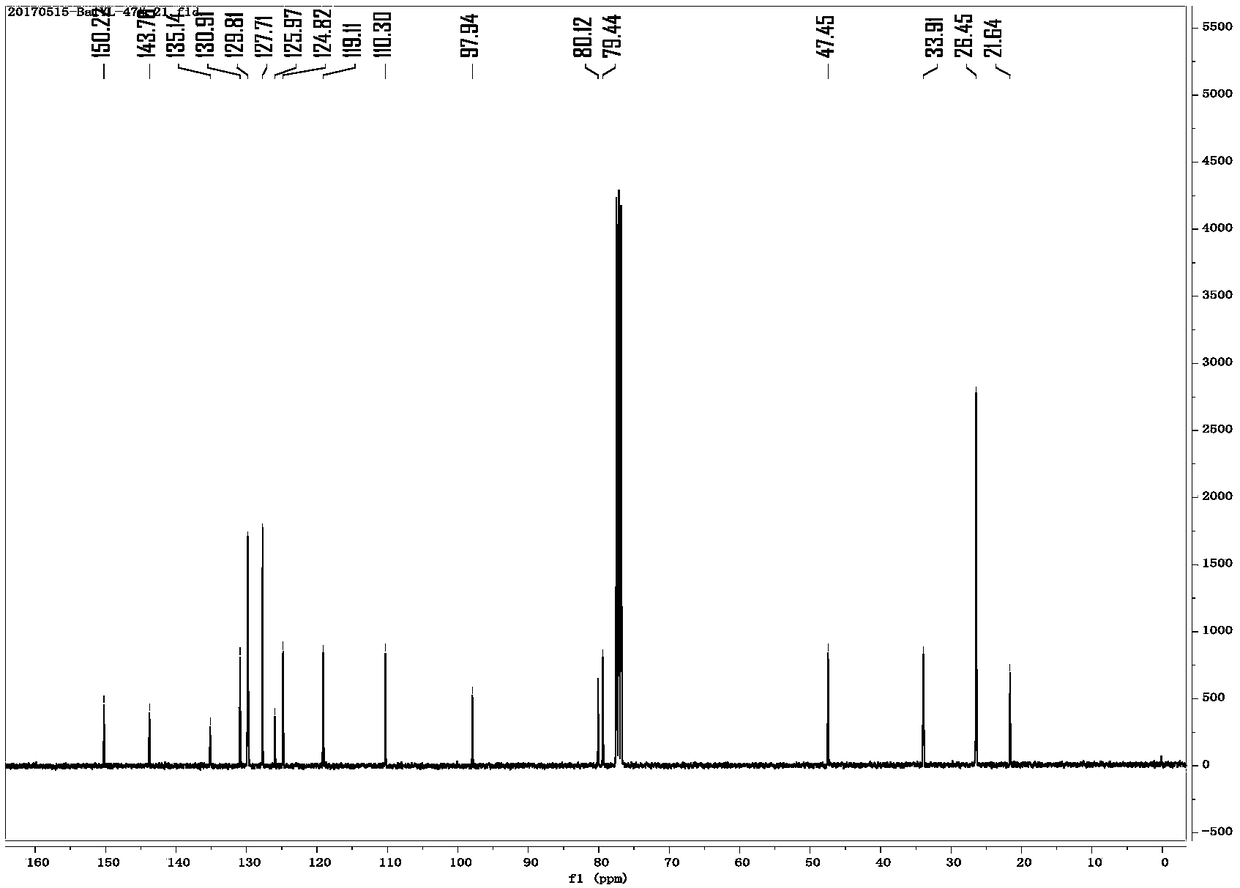
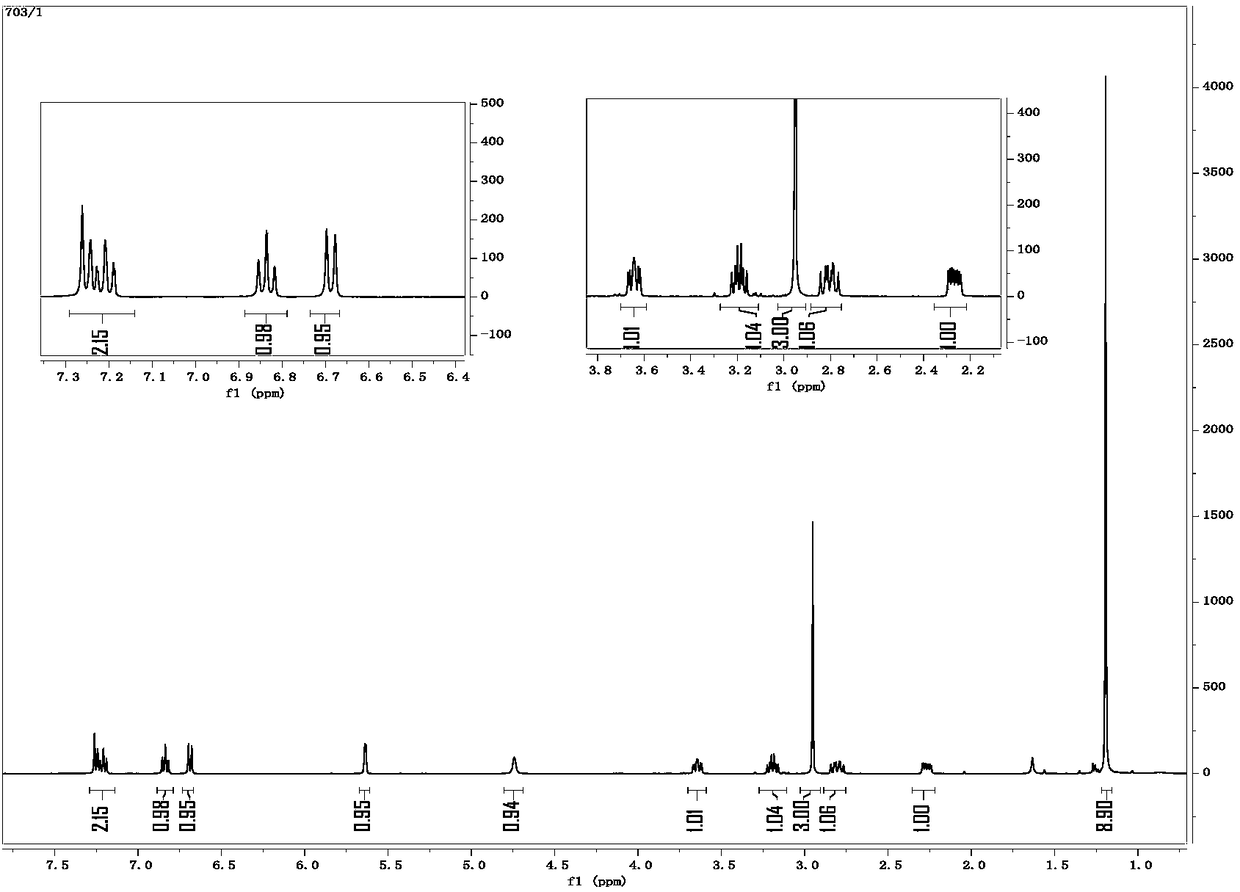
Image
Examples
preparation example Construction
[0044] Specifically, the preparation method comprises the following steps:
[0045] (1) With substrate I and substrate II as starting materials, under the catalysis of small organic molecule catalysts, react in a solvent to generate the first product; wherein, substrate I has a structural formula as shown in formula (1) , substrate II acts as oxidizing agent simultaneously, and described first product has the structural formula shown in formula (two):
[0046]
[0047]Catalyst is organic iodide such as NaI, KI and TBAI (tetraethyl ammonium iodide) etc., is preferably organic small molecule TBAI (tetraethyl ammonium iodide), and the molar consumption of catalyzer is 10-30% of substrate I, Preferably it is 20%. Substrate II, the oxidizing agent, is TBHP (tert-butyl alcohol peroxide) or CHP (cumyl hydroperoxide). The substrate II of the present invention simultaneously acts as an oxidizing agent and reacts with a catalyst. The molar ratio of substrate I and substrate II is ...
Embodiment 1
[0061] Tetrabutylammonium iodide (0.04mmol, 14.8mg), N-(2-(1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide (0.2mmol, 62.9mg), 8ml of 1,4-dioxane, and finally tert-butanol peroxide (0.8mmol). The reaction was heated to 90°C for 2 hours. The reaction was cooled to room temperature, sodium borohydride (0.6 mmol, 22.7 mg) was added in portions, and stirred for 30 minutes. Add saturated NH to the reaction system 4 Cl solution was stirred for 3 min and extracted three times with EtOAc in a separatory funnel. The above EtOAc solution was washed three times with saturated sodium thiosulfate solution, the organic phase was dried over anhydrous sodium sulfate, filtered and the organic phase was concentrated under reduced pressure to remove the solvent to obtain the crude product. The crude product was separated with a silica gel column, eluent petroleum ether \ ethyl acetate = 30:1. The target product was collected, and the organic phase was concentrated under reduced pressure to re...
Embodiment 2
[0066] Tetrabutylammonium iodide (0.04mmol, 14.8mg), N-(2-(1H-indol-3-yl)ethyl)methanesulfonamide (0.2mmol, 47.7 mg), 8 ml of 1,4-dioxane, and finally tert-butanol peroxide (0.8 mmol). The reaction was heated to 90°C for 2 hours. The reaction was cooled to room temperature, sodium borohydride (0.6 mmol, 22.7 mg) was added in portions, and stirred for 30 minutes. Add saturated NH to the reaction system 4 Cl solution was stirred for 3 min and extracted three times with EtOAc in a separatory funnel. The above EtOAc solution was washed three times with saturated sodium thiosulfate solution, the organic phase was dried over anhydrous sodium sulfate, filtered and the organic phase was concentrated under reduced pressure to remove the solvent to obtain the crude product. The crude product was separated with a silica gel column, eluent petroleum ether \ ethyl acetate = 30:1. The target product was collected, and the organic phase was concentrated under reduced pressure to remove t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com