A process for preparing 2,3,5-trimethyl-p-benzoquinone by catalytic oxidation
A catalytic oxidation and trimethylation technology, applied in the preparation of oxidized quinone, organic chemistry, etc., can solve the problems of slow oxidation reaction, low oxygen utilization rate, and immature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
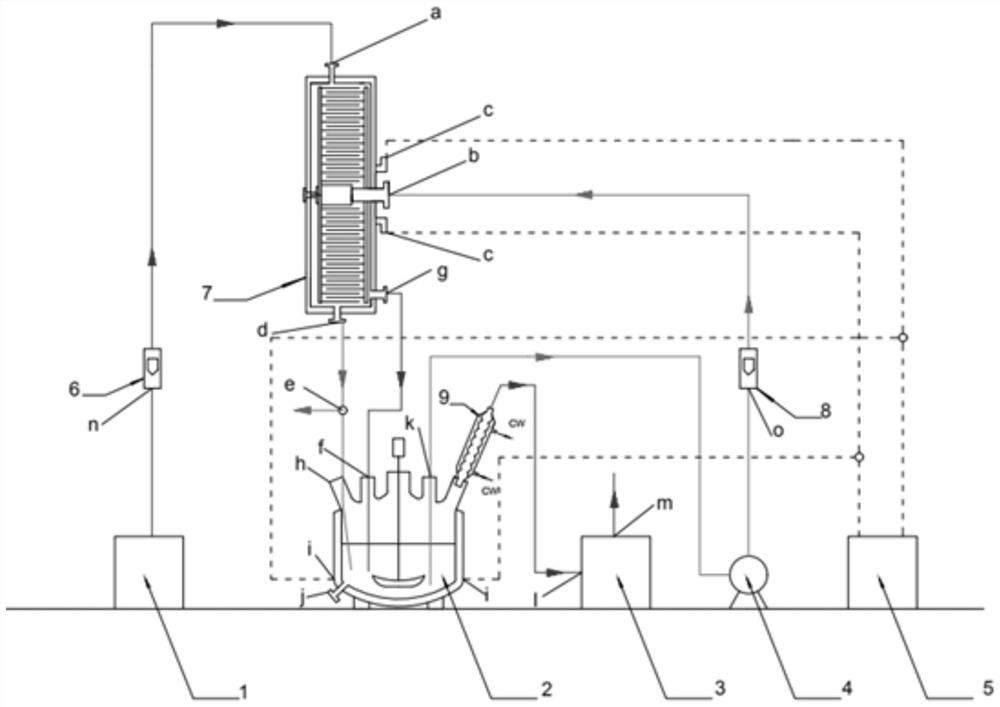
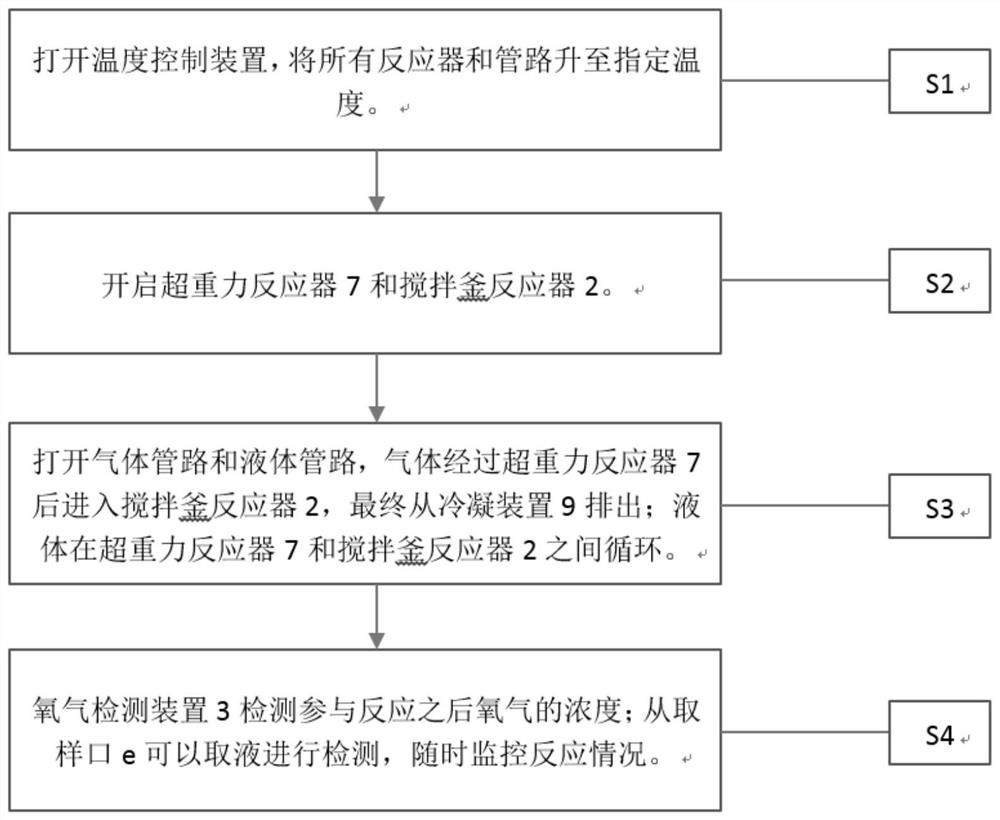
[0022] According to above narration, the present invention proposes the preparation method of a set of 2,3,5-trimethyl-p-benzoquinone matched with the preparation technology of 2,3,5-trimethyl-p-benzoquinone of the present invention, now In conjunction with the preparation process of the above-mentioned 2,3,5-trimethyl-p-benzoquinone to understand the idea of the preparation method of 2,3,5-trimethyl-p-benzoquinone, see figure 2 . The preparation technology of this 2,3,5-trimethyl-p-benzoquinone comprises the following steps:
[0023] S1: Turn on the temperature control device and raise all reactors and pipelines to the specified temperature.
[0024]S2: Turn on the supergravity reactor 7 and the stirred tank reactor 2, and adjust to the required speed.
[0025] S3: Open the gas pipeline and the liquid pipeline, the gas enters the stirred tank reactor 2 after passing through the supergravity reactor 7, and finally discharges from the condensation device 9; the liquid circ...
Embodiment 1
[0034] Embodiment 1: Effect experiment of supergravity level
[0035] The reaction temperature is 73°C, the volume fraction of the water phase in the liquid phase is 0.83, the rotation speed of the stirred tank reactor is 600rpm, the concentration of 2,3,6-trimethylphenol in the oil phase is the same as that of CuCl in the water phase 2 The concentration ratio is 1:2, the above refers to the molar concentration ratio, CuCl 2 The concentration of the aqueous solution is 1mol / L, the gas-liquid volume flow ratio is 2.6, and the result of reaction for 10 minutes is as follows:
[0036] Hypergravity level (g) Yield of quinone (%) 30 14.14 120 15.34 270 15.62 480 16.9 750 15.06 Stirred tank reactors are used separately under the same conditions 5.64
[0037] The above results show that, compared with using the stirred tank reactor alone, the yield of 2,3,5-trimethyl-p-benzoquinone can be increased by about 10% after adding the high gra...
Embodiment 2
[0038] Example 2: Gas volume influence experiment
[0039] The reaction temperature is 75°C, the volume fraction of the water phase in the liquid phase is 0.83, the rotation speed of the stirred tank reactor is 600rpm, the supergravity level of the supergravity reactor is 270g, the liquid flow rate is 20L / h, and 2,3,6-trimethanone in the oil phase Base phenol concentration and CuCl in aqueous phase 2 The concentration ratio is 1:2, the above refers to the molar concentration ratio, CuCl 2 The concentration of the aqueous solution is 1mol / L, and the result of reacting for 10 minutes is as follows:
[0040]
[0041]
[0042] The above results show that, compared with using the stirred tank reactor alone, the yield of 2,3,5-trimethyl-p-benzoquinone can be increased by about 9-13% after adding the high gravity reactor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com