Isoniazide ramification and preparation method and application thereof
A technology of isoniazid and its derivatives, applied in the field of organic chemical synthesis, can solve the problems of high production cost and weak reactivity of ketone carbonyl, and achieve the effects of low production cost, easy preparation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 0.0137g (0.1mmol) of isoniazid and 0.0234g (0.1mmol) of camphorsulfonic acid into a 25mL Erlenmeyer flask, add 3mL of a mixed solvent of ethanol and acetonitrile (v:v=1:1), shake to make it fully dissolve. After standing at room temperature for 2-3 days, 0.0358 g of colorless transparent crystals was obtained with a yield of 96.5%.
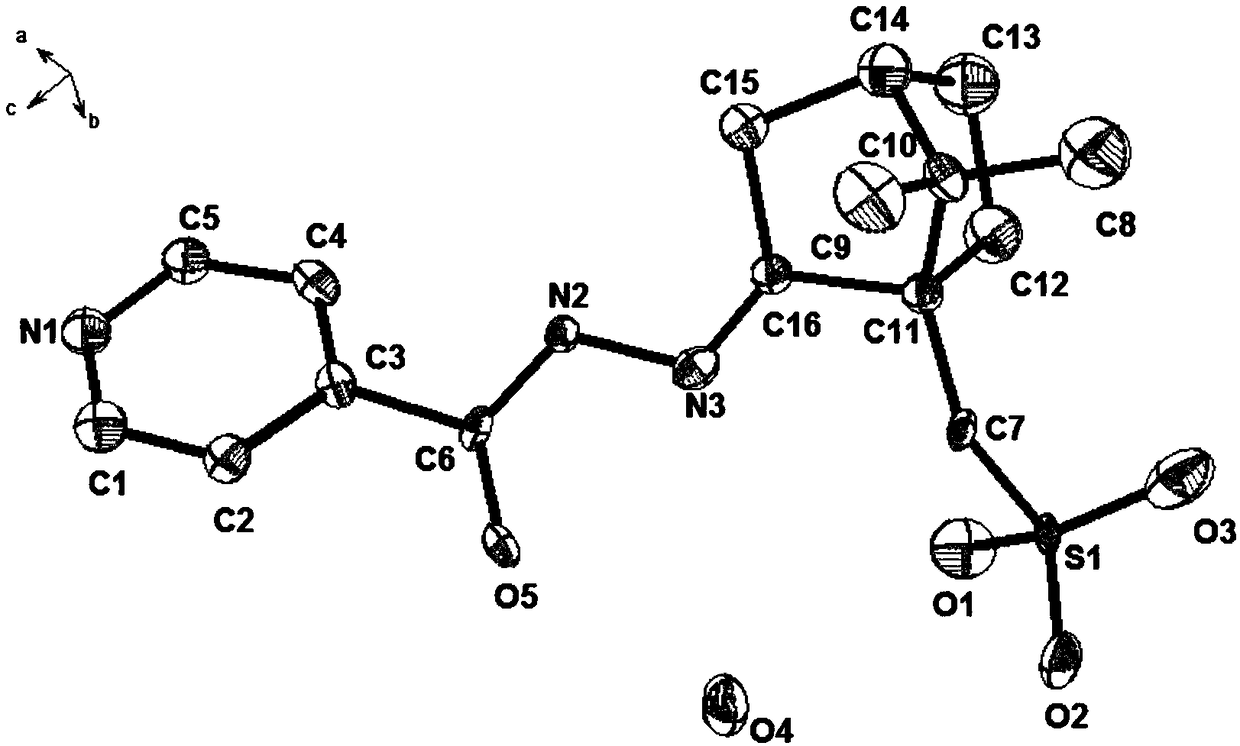
[0026] The crystals of the target product were analyzed by X-ray single crystal diffraction. Monochromatization of Mo Kα using graphite on a Bruker D8-ray diffractometer Radiation is used as a diffraction light source to collect diffraction intensity data for single crystals. The three-dimensional structure shows that the amino group of isoniazid and the ketone carbonyl group of camphorsulfonic acid form a C=N double bond after dehydration condensation Indicates the generation of target compounds, such as figure 1 shown.
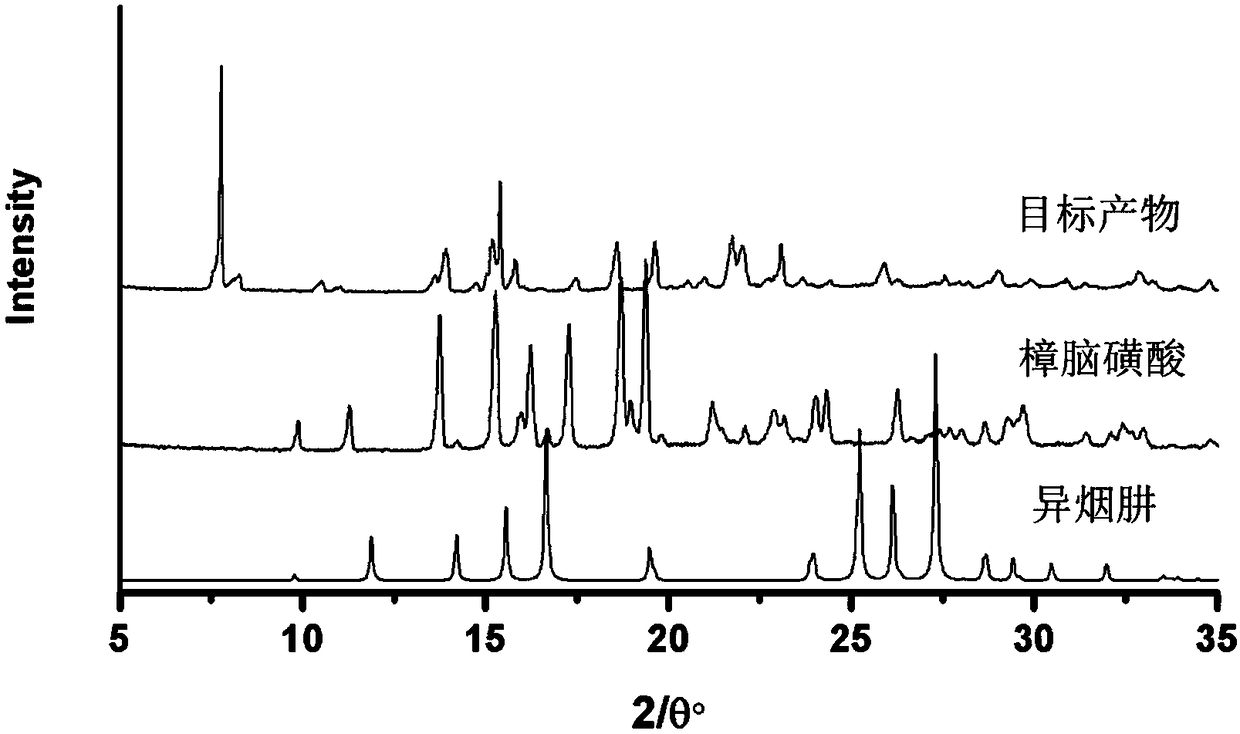
[0027] The target product was analyzed by X-ray powder diffraction, such as figure 2 shown. The crystals ...
Embodiment 2
[0031] Add 0.0137g (0.1mmol) of isoniazid and 0.0234g (0.1mmol) of camphorsulfonic acid into a 25mL Erlenmeyer flask, add 3mL of a mixed solvent of methanol and acetonitrile (v:v=1:1), shake to make it fully dissolve. After standing at room temperature for 2-3 days, 0.0342 g of colorless transparent crystals was obtained with a yield of 92.2%.
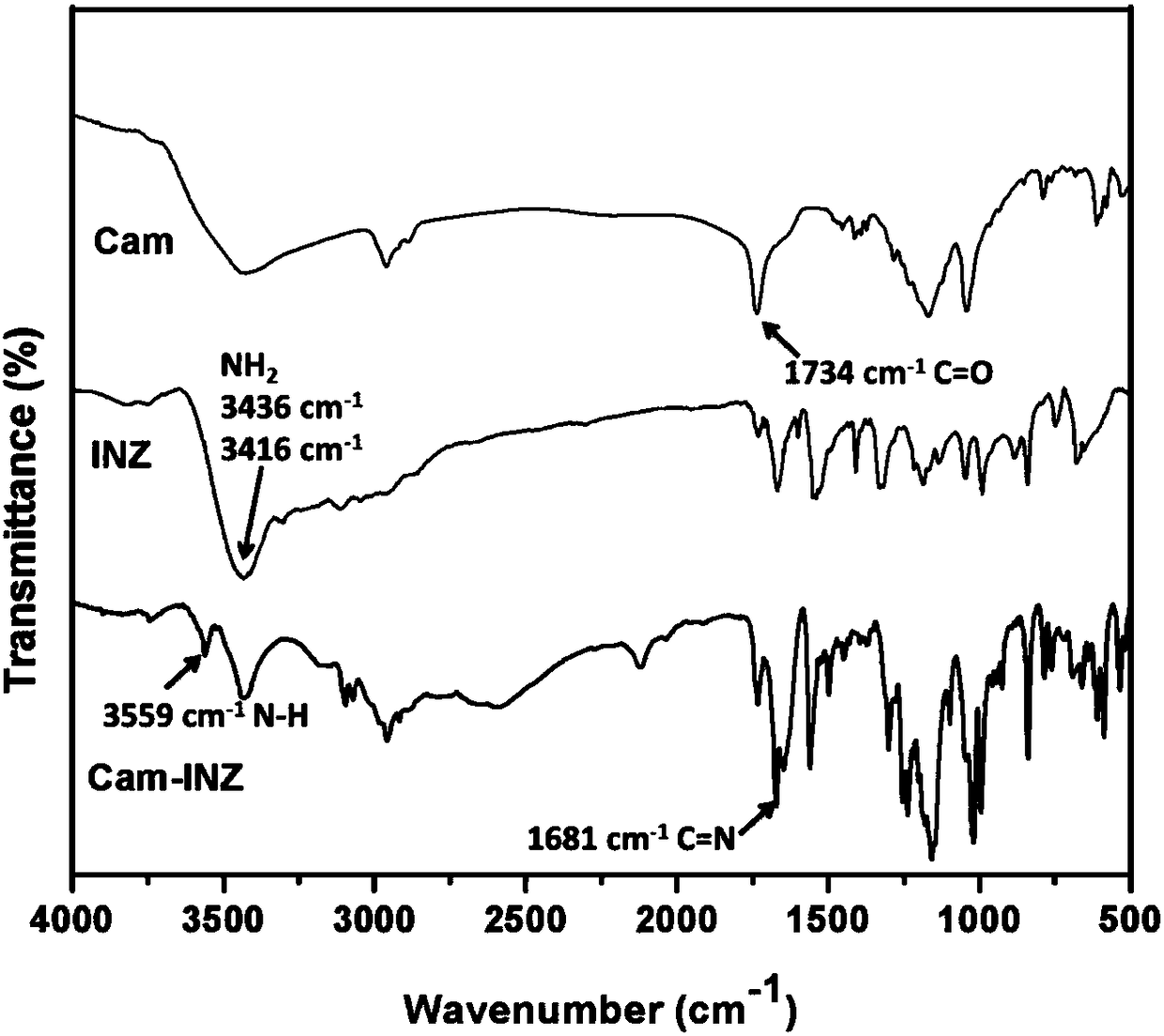
[0032] The product was analyzed by X-ray single crystal diffraction, X-ray powder analysis and infrared analysis, confirming that the target product 2-(2-isonicotinylhydrazono)-7,7-dimethylbicyclo[2.2.1]heptane was synthesized Alk-1-yl-methanesulfonic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com