A kind of method of lithium hydride selective reduction chloroalkyl chlorosilane
A technology of chloroalkylchlorosilane and chloroalkylhydrosilane, which is applied in the field of selective reduction of chloroalkylchlorosilane by lithium hydride, can solve the problems that the rapid and efficient selective reduction of chloroalkylchlorosilane cannot be achieved, and achieve the reduction reaction The effect of fast rate, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] Specifically, the preparation method of above-mentioned chloroalkyl hydrosilane comprises the following steps:
[0049] Step S1: under anhydrous conditions and under the protection of an inert gas, mix lithium hydride, catalyst and ether solvent, and stir evenly to obtain a mixed solution of lithium hydride and catalyst;
[0050] Step S2: Under stirring, add chloroalkylchlorosilane dropwise to the mixed solution of lithium hydride and catalyst, carry out the reduction reaction at 0°C-140°C for 0.1h-24h, and the reaction product is separated and purified (for example, distillation) Finally, the corresponding chloroalkylhydrosilanes are obtained.
[0051] Above-mentioned chloroalkyl chlorosilane can be ClPrSiMeCl 2 , ClCH 2 SiMeCl 2 , ClCH 2 SiMe 2 Cl, Cl 2 CHSiMeCl 2 , ClCH 2 SiCl 3 , ClCH 2 CH 2 SiMeCl 2 or Cl 2 CHSiCl 3 . It can be seen from this that the above method for preparing chloroalkylhydrosilanes can be applied to the reduction reaction of vario...
Embodiment 1
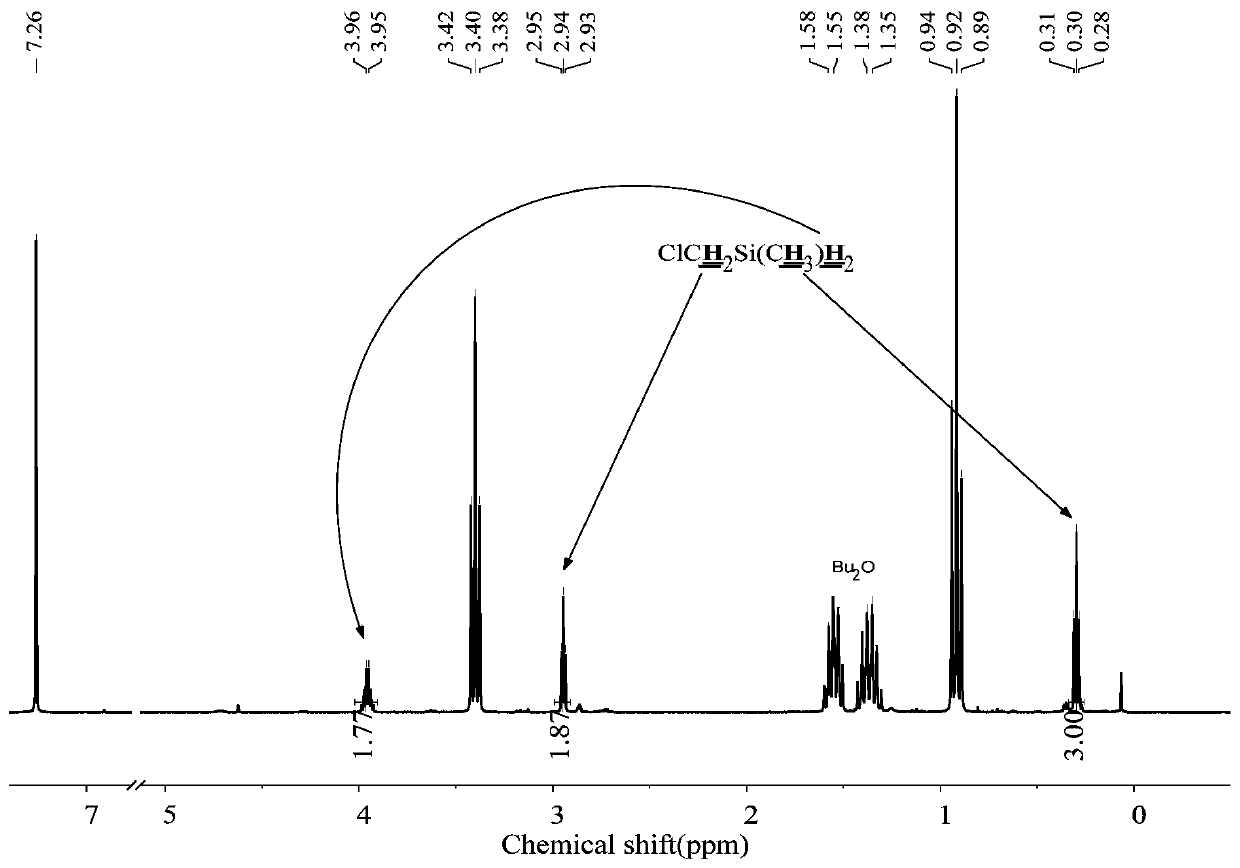
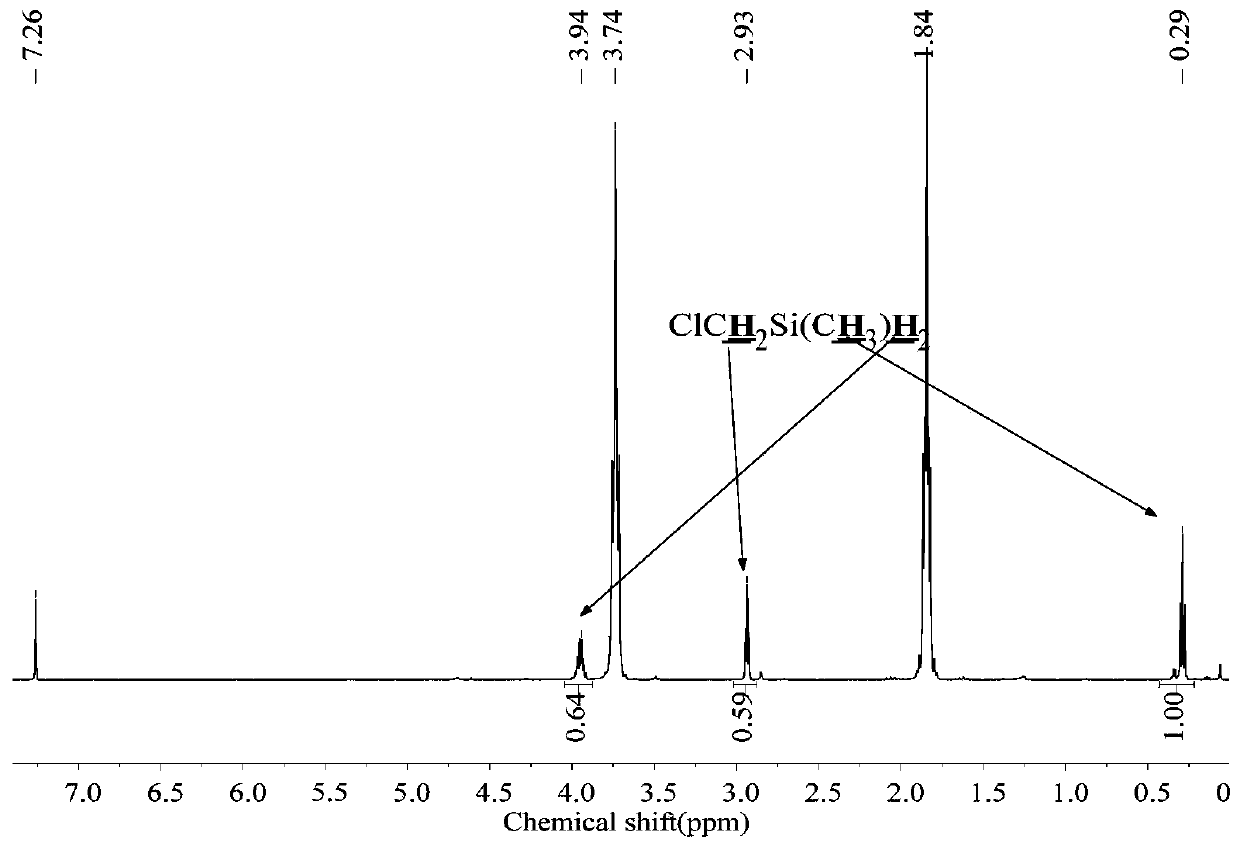
[0060] The 250mL three-neck round bottom flask was dried, connected with a mechanical stirrer, spherical condenser, constant pressure funnel, and gas guiding device, and then evacuated for three times to replace nitrogen, and at the same time baked with a heat gun to remove attached water vapor. Under a nitrogen atmosphere, add 1.8g (0.22mol) LiH, 0.022g (0.001mol) LiBH to the reaction flask 4 , 20mL tetrahydrofuran solvent, and control the reaction temperature at 30-40°C. Add 16.7g (0.1mol) ClCH to the constant pressure funnel 2 SiMeCl 2 , slowly dropwise into the reaction flask under mechanical stirring. Continue to stir the reaction for 1h after the dropwise addition, the reduction product is ClCH 2 SiMeH 2 , the yield was 100%.
[0061] The method of the present embodiment is reacted to the product, raw material and solvent in the final system 1 H-NMR spectrum as figure 2 shown.
Embodiment 2
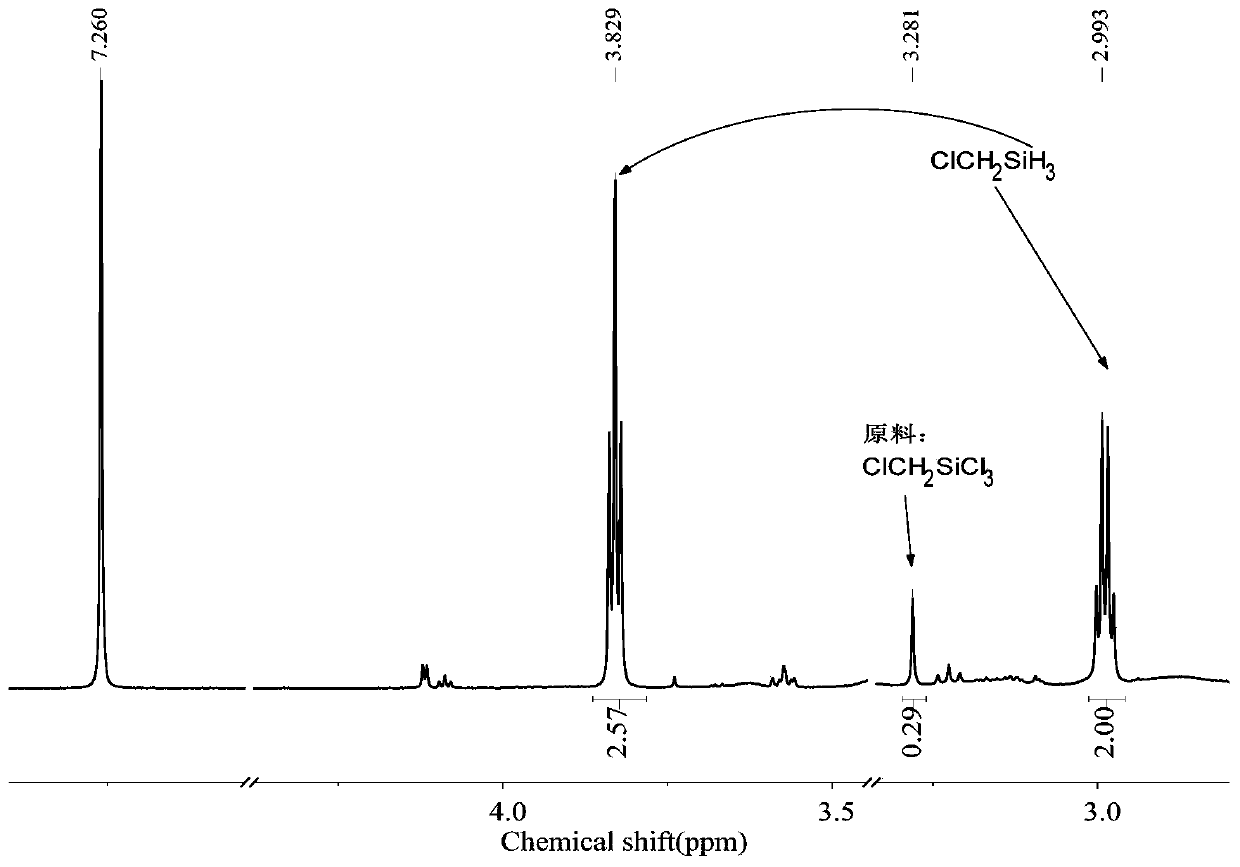
[0067] The 250mL three-neck round bottom flask was dried, connected with a mechanical stirrer, a spherical condenser, a constant pressure funnel and a gas guiding device, vacuumed three times to replace nitrogen, and at the same time baked with a hot air gun to remove attached water vapor. Under a nitrogen atmosphere, add 2.7g (0.33mol) LiH, 0.022g (0.001mol) LiBH to the reaction flask 4 , 20mL tetrahydrofuran solvent. Add 18.4g (0.1mol) ClCH to the constant pressure funnel 2 SiCl 3 , slowly added dropwise into the reaction flask under mechanical stirring, keeping the reaction temperature between 30 and 40°C. After the dropwise addition was completed, the reaction was continued for 1 h to obtain the product ClCH 2 SiH 3 , the yield was 100%.
[0068] The method of the present embodiment is reacted to the product, raw material and solvent in the final system 1 H-NMR spectrum as Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com