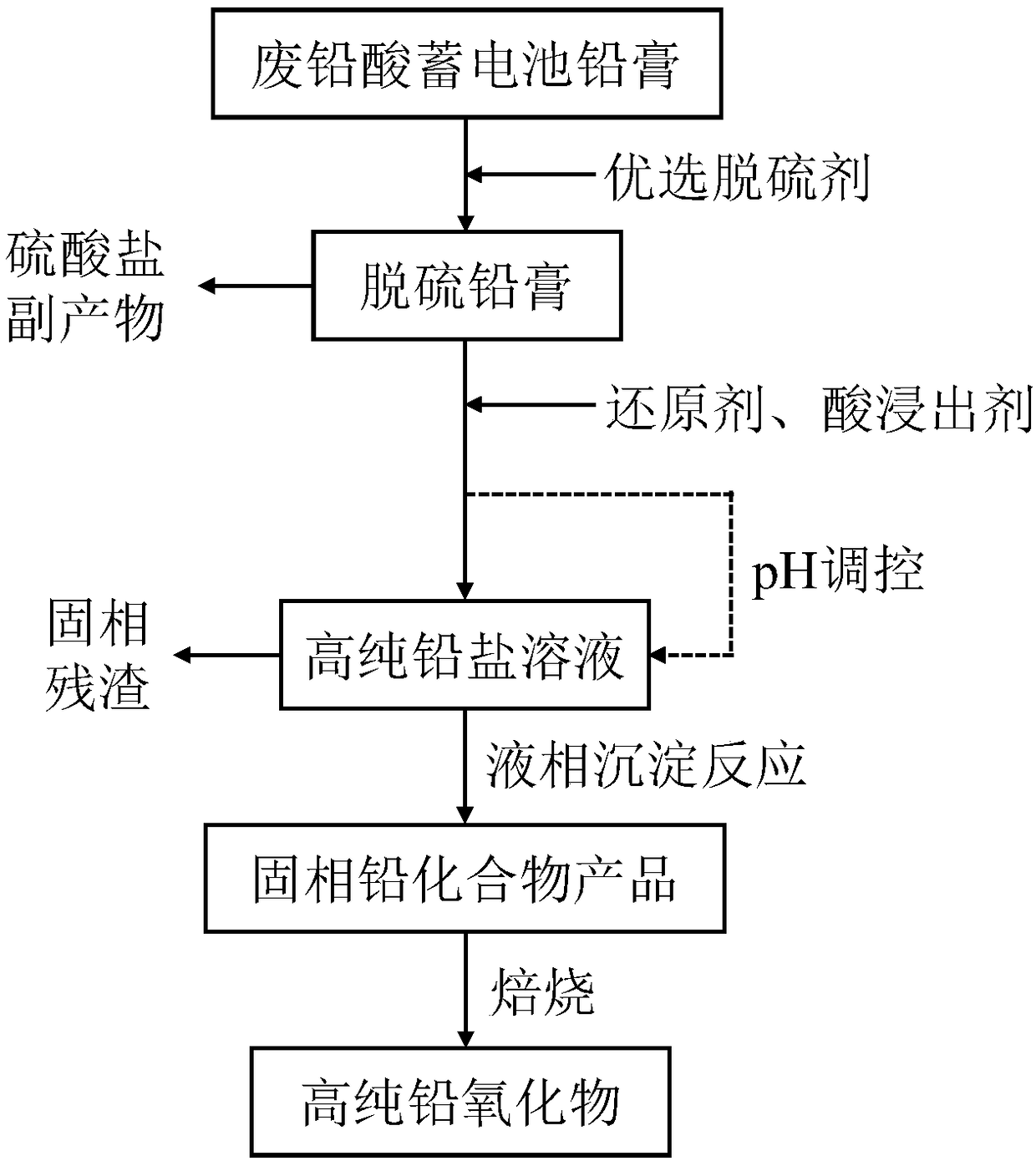
Method for preparing high-purity lead compound through wet recovery and impurity removal of waste lead plaster
A technology of lead compounds and waste lead paste, which is applied in the field of preparing high-purity lead compounds, can solve the problems of high content of Ba and Fe elements, high content of impurities, and difficult removal, and achieve controllable process, high purity, and time-consuming process short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] To desulfurize the waste lead paste, add 200g waste lead paste to 400mL sodium hydroxide solution with a concentration of 2.05mol / L at a uniform speed, stir and react for 10min, filter and dry to obtain 165g desulfurized lead paste. The desulfurized lead paste is leached by nitric acid. Take 100g of desulfurized lead paste and add it to 500mL leachant solution at a uniform speed, which contains reducing agent H 2 o 2 The concentration is 1.06mol / L, the leaching agent HNO 3 The concentration is 1.87mol / L. Stir the reaction for 10 minutes, then add ammonia water to adjust the pH to 3.68, filter to obtain a pure lead nitrate solution, and measure its concentration to be 0.8442mol / L. Get 100mL of lead nitrate solution and dilute it to 1000mL, add 100mL of 1.266mol / L ammonium carbonate solution therein, stir and react for 30min, filter and dry to obtain lead carbonate, its XRD pattern is as follows: Figure 4 shown. It was calcined at 550°C for 4 hours in an air atmosphe...
Embodiment 2
[0061] To desulfurize the waste lead paste, add 200g waste lead paste to 2000mL sodium hydroxide solution with a concentration of 0.62mol / L at a uniform speed, stir and react for 30min, filter and dry to obtain 163g desulfurized lead paste. The desulfurized lead paste is leached by acetic acid. Take 100g of desulfurized lead paste and add it to 370mL leachant solution at a uniform speed, which contains reducing agent H 2 o 2 The concentration of leaching agent acetic acid is 1.43mol / L, and the concentration of acetic acid is 2.51mol / L. Stir the reaction for 300 min, filter to obtain a pure lead acetate solution with a pH of 4.31, and measure its concentration to be 0.8581 mol / L. Take 100mL of lead acetate solution and dilute to 1000mL, add 100mL of 1.287mol / L ammonium carbonate solution to it, stir and react for 60min, filter and dry to obtain lead carbonate product.
Embodiment 3
[0063] To desulfurize the waste lead paste, add 200g waste lead paste to 900mL sodium hydroxide solution with a concentration of 1.41mol / L at a uniform speed, stir and react for 30min, filter and dry to obtain 167g desulfurized lead paste. The desulfurized lead paste is leached by nitric acid, and 100g of desulfurized lead paste is added to 1000mL of leaching agent solution at a uniform speed, which contains reducing agent H 2 o 2 The concentration is 0.365mol / L, the leaching agent HNO 3 The concentration is 0.873mol / L. Stir the reaction for 30 minutes, then add 40wt.% sodium hydroxide solution to adjust the pH to 3.50, filter to obtain a pure lead nitrate solution, and determine its concentration to be 0.4258mol / L. Take 250mL of lead nitrate solution and dilute it to 1000mL, add 500mL of 0.426mol / L sodium carbonate solution to it, stir and react for 30min, filter and dry to obtain lead carbonate product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com