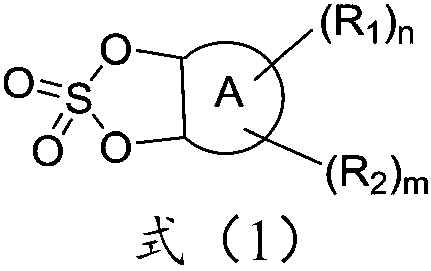
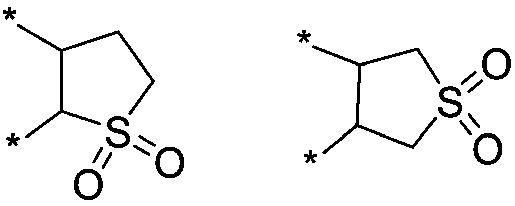
Additive for non-aqueous electrolyte of lithium ion battery and preparation method and application thereof
A non-aqueous electrolyte, lithium-ion battery technology, applied in non-aqueous electrolyte storage batteries, electrolyte storage battery manufacturing, secondary batteries, etc. Number of cycles, reduction of increase in membrane impedance, effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
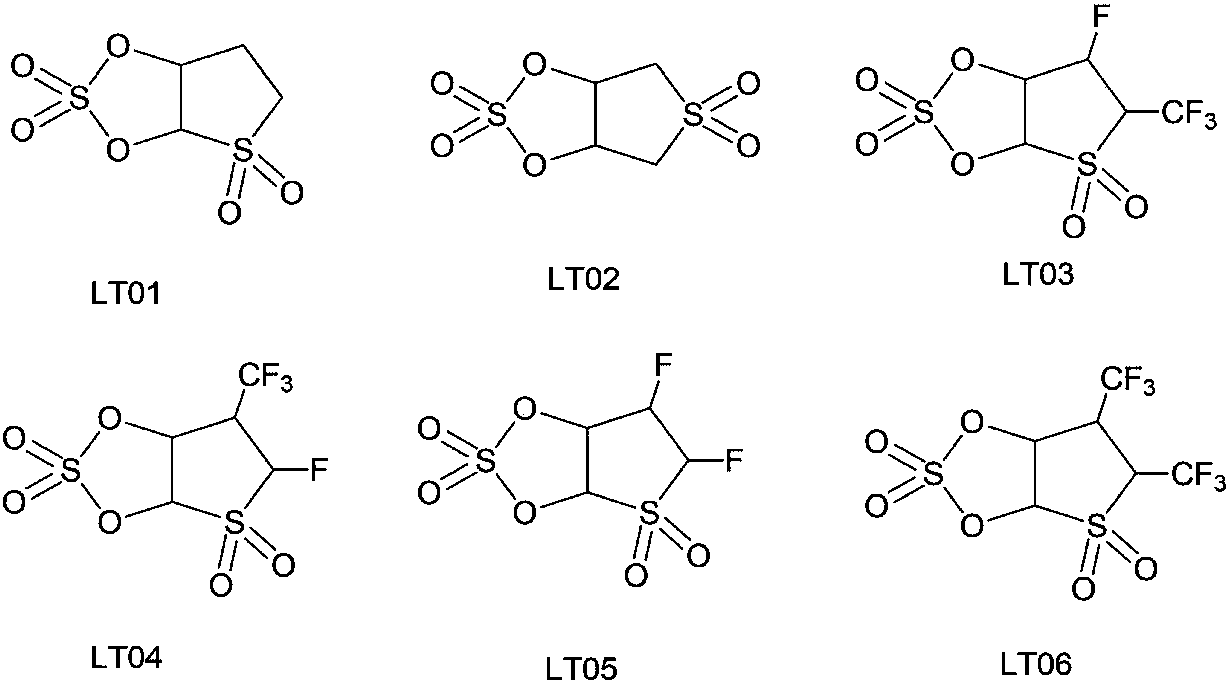
[0035] Embodiment 1: the synthesis of compound LT01:
[0036] Weigh 76.0 g (0.5 mol) of 2,3-dihydroxysulfolane and 250 mL of dichloroethane into a 1 L three-necked flask. Under nitrogen protection, 71.5 g (0.6 mol) of thionyl chloride was slowly added dropwise to the reaction system at 20-25° C., and the drop was completed in about 1.0 hr. Keep warm at 25-30°C for 2.5hrs.
[0037] Under the condition of 25-30° C., the above reaction solution was dropped into a mixed solution containing 0.1035 g (0.0005 mol) of ruthenium trichloride trihydrate and 150 g of 8% sodium bicarbonate (0.12 mol) aqueous solution. 323.3 g of sodium hypochlorite (0.50 mol) aqueous solution was added dropwise at 0-5°C. After about 3.0hrs of dripping, keep warm at 0~5℃ for 1.0hr.
[0038] Add saturated sodium bisulfite aqueous solution dropwise to the reaction system to quench the reaction. The layers were separated, and the organic phase was dried over anhydrous sodium sulfate. Pass through the colu...
Embodiment 2
[0039] Embodiment 2: the synthesis of compound LT02
[0040] Weigh 76.0 g (0.5 mol) of 3,4-dihydroxysulfolane and 250 mL of dichloromethane into a 1 L three-necked flask. Under nitrogen protection, 64.5 g (0.525 mol) of thionyl chloride was slowly added dropwise to the reaction system at 15-20° C., and the drop was completed in about 1.0 hr. Keep warm at 20-25°C for 2.0hrs.
[0041] Under the condition of 25-30° C., the above reaction solution was dropped into a mixed solution containing 0.1035 g (0.0005 mol) ruthenium trichloride trihydrate and 150 g potassium carbonate (0.10 mol) aqueous solution with a mass fraction of 9.3%. 388g of sodium hypochlorite (0.6mol) aqueous solution was added dropwise at 0-5°C. After about 3.0hrs of dripping, keep warm at 0~5℃ for 0.5hr.
[0042] Add saturated sodium bisulfite aqueous solution dropwise to the reaction system to quench the reaction. The layers were separated, and the organic phase was dried over anhydrous sodium sulfate. Pas...
Embodiment 3
[0043] Embodiment 3: the synthesis of compound LT03
[0044]Weigh 119.0 g (0.5 mol) of 3-F-4,5-dihydroxy-2-trifluoromethyl sulfolane and 250 mL of dichloromethane into a 1 L three-neck flask. Under nitrogen protection, 64.5 g (0.525 mol) of thionyl chloride was slowly added dropwise to the reaction system at 15-20° C., and the drop was completed in about 1.0 hr. Keep warm at 20-25°C for 2.0hrs.
[0045] Under the condition of 25-30 DEG C, the above reaction solution was dropped into a mixed solution containing 0.029g (0.00006mol) manganese acetate tetrahydrate and 150g aqueous solution of sodium carbonate (0.21mol) with a mass fraction of 15.0%. 200g of hydrogen peroxide (0.6mol) aqueous solution was added dropwise at 0-5°C. After about 3.0hrs of dripping, keep warm at 0~10℃ for 4.0hr.
[0046] Add saturated sodium bisulfite aqueous solution dropwise to the reaction system to quench the reaction. The layers were separated, and the organic phase was dried over anhydrous sod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com