SN38 lipid composition, and preparation method and application thereof
A technology of lipid composition and phospholipids, which is applied in the direction of drug combination, liposome delivery, drug delivery, etc., can solve the problems of difficult industrialized large-scale production, complex preparation process, and low drug encapsulation efficiency, so as to reduce toxic and side effects, Effects of prolonging circulation time and overcoming multidrug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of SN38 lipid composition freeze-dried powder injection
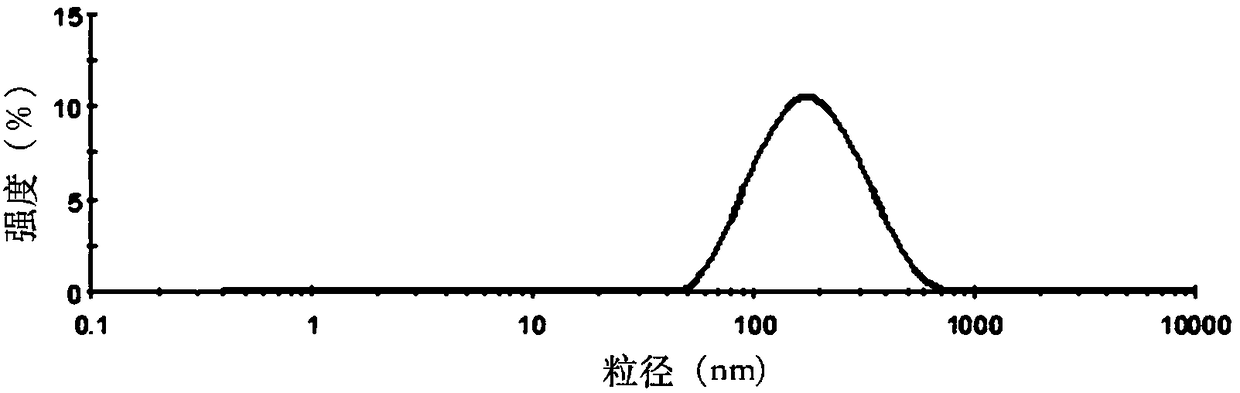
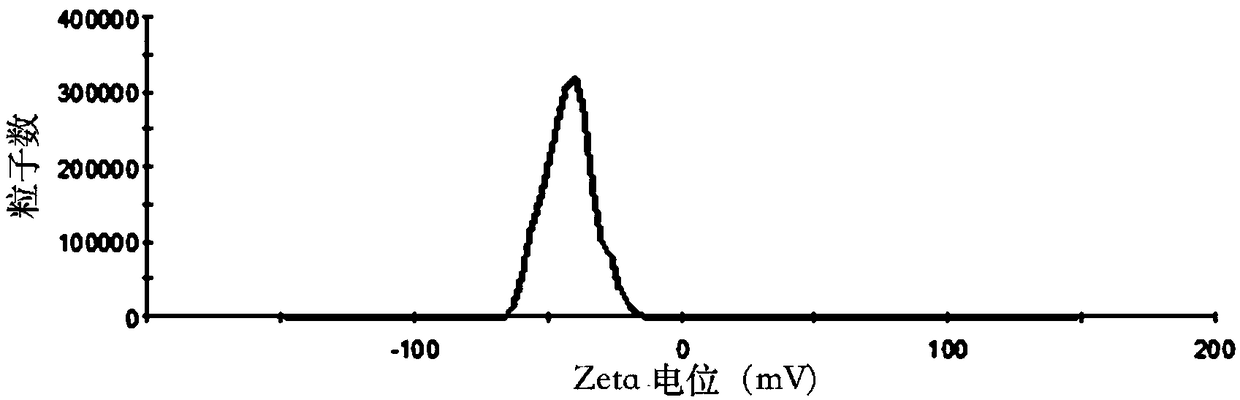
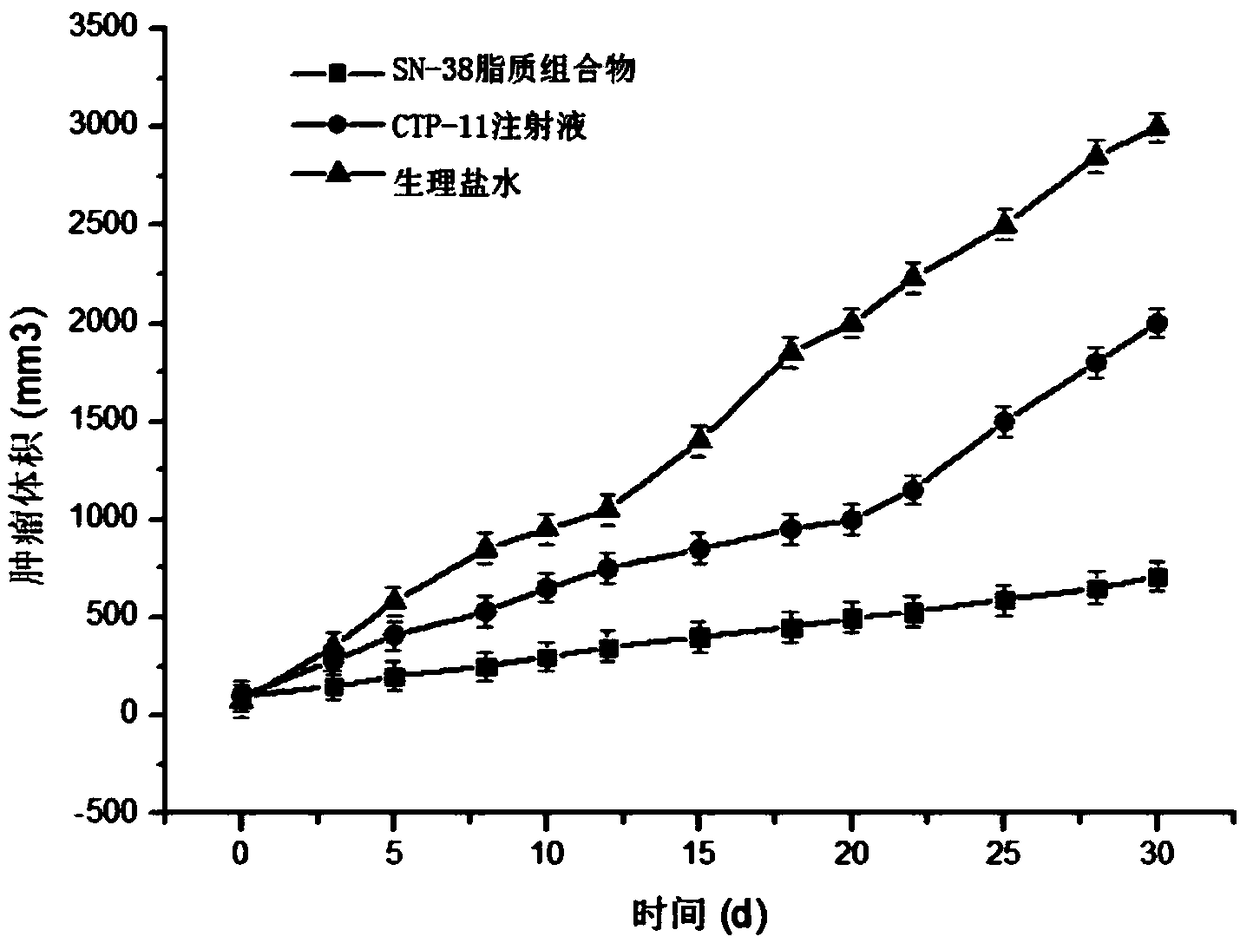
[0049] Weigh 30mg of SN38, 600mg of soybean lecithin, 60mg of soybean oil, 150mg of cholesterol and 60mg of PEG-DSPE into a 250mL round-bottomed flask, dissolve in 30mL of chloroform:methanol (1:1, v / v), and store at 60-70°C Evaporate under reduced pressure to form a lipid film on the bottle wall, add 30mL of PBS solution (pH=5) containing 10wt% sucrose and 0.1wt% poloxamer 188 to hydrate for 1h to fully hydrate the film, and then homogenize under high pressure (homogeneous Mass pressure 20000psi) 5 times to obtain the lipid composition suspension, which is sub-packed into vials, and freeze-dried to obtain the SN38 lipid composition freeze-dried powder. The freeze-dried SN38 lipid composition freeze-dried powder injection after freeze-drying adds water reconstitution, measures its particle size, electric potential and encapsulation efficiency, its particle size of the result (see figure 1 )...
Embodiment 2
[0050] Example 2 Preparation of SN38 Lipid Composition Freeze-dried Powder
[0051] Weigh 30 mg of SN38, 1200 mg of hydrogenated soybean lecithin, 100 mg of MCT, 200 mg of cholesterol, 50 mg of DSPG, 50 mg of PEG-DSPE, and 50 mg of HS15 in a 100 mL round-bottomed flask, dissolve them in 50 mL of chloroform / methanol (9:1, v / v) mixed solvent, Evaporate under reduced pressure at 60°C to form a lipid film on the bottle wall, add 30mL sodium acetate buffer (pH=3) containing 15wt% sucrose and 0.5wt% PVP-K30 to hydrate for 2 hours, and then homogenize under high pressure to obtain lipid The substance composition suspension is divided into vials and freeze-dried to obtain the product. The freeze-dried phospholipid composition freeze-dried powder was reconstituted with water, and its particle size and encapsulation efficiency were measured. The results showed that the particle size and encapsulation efficiency were 178.2nm and 86.7% respectively.
Embodiment 3
[0052] The preparation of embodiment 3 SN38 lipid composition
[0053] Weigh 30mg of SN38, 335mg of egg yolk lecithin, 10mg of camellia oil, 30mg of TPGS, 50mg of cholesterol and 15mg of PEG-DSPE into a 100mL round bottom flask, and dissolve in 50mL of dichloromethane / methanol (1:1, v / v) mixed solvent Afterwards, spray-dry (inlet temperature: 60°C) to obtain white particles, add 60mL of 10wt% trehalose, 0.5wt% chitosan sodium succinate buffer (pH=4) for hydration for 2 hours, and then homogenize under high pressure A lipid composition suspension is obtained. The particle size and encapsulation efficiency were determined to be 120.8nm and 85.4%, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com