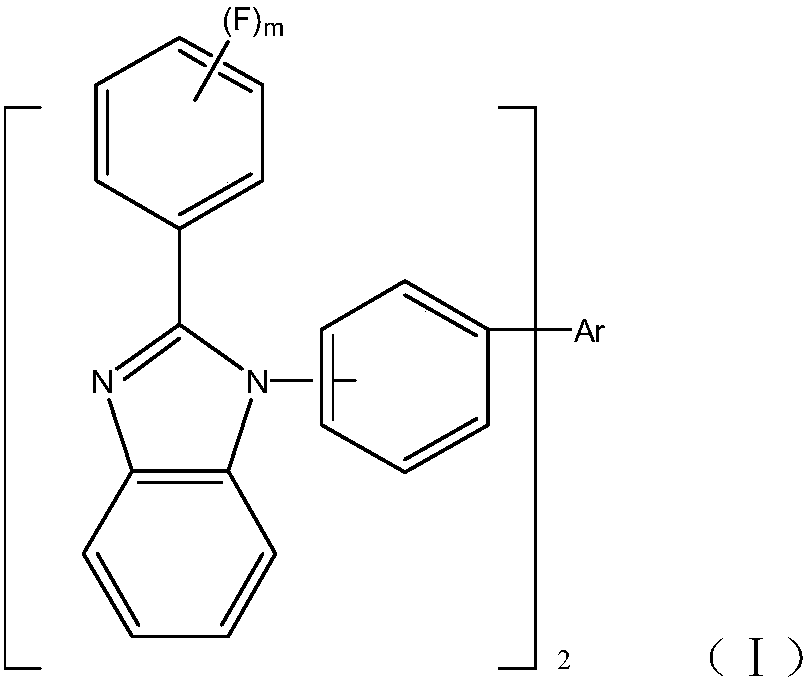
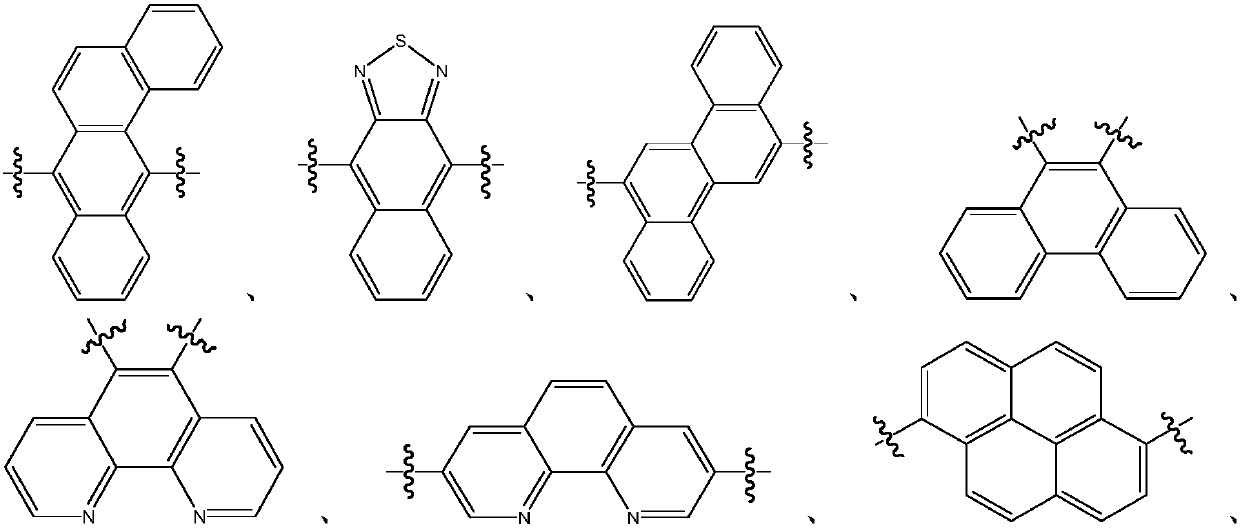
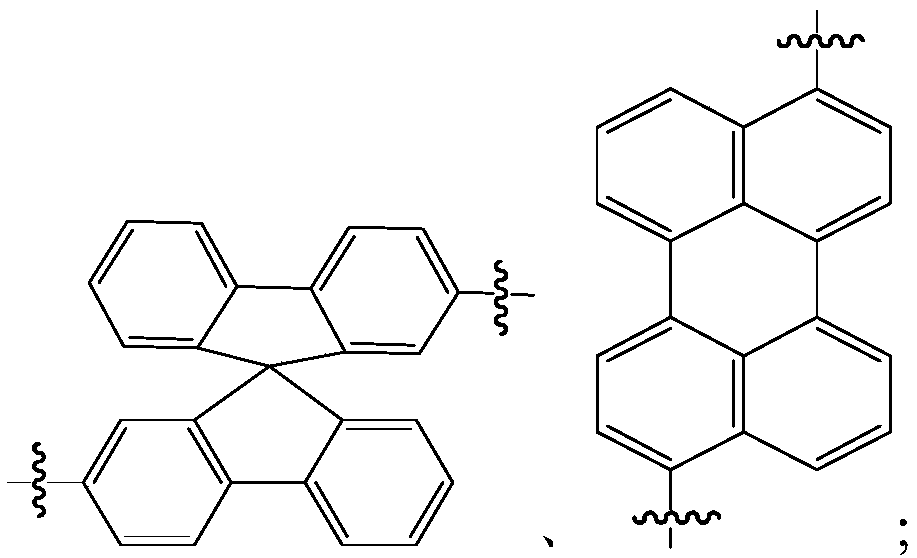
Organic material and applications of organic material in organic electroluminescent devices
A technology of organic materials and organic light-emitting layers, applied in the field of organic electroluminescence display, can solve problems such as small molecular weight, easy crystallization of materials, and low glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Synthesis of (Compound 1)
[0092] The synthetic route is as follows:
[0093]
[0094] 1) Synthesis of compound 1-1
[0095] A 1000 ml three-necked flask was equipped with a magnetic stirrer. After argon replacement, 26.49 g of 1-bromo-4-iodobenzene (purity 99%, 0.094 mol) and THF 500 ml were added in sequence according to the above amounts. Add 38ml of n-BuLi (concentration 2.5M, 0.095mol) dropwise at -90°C, and then add 8.4g of anthraquinone (purity 99%, 0.04mol). 20h. Add 500m water for hydrolysis, separate the liquid, extract the water phase with dichloromethane, combine the organic layers, evaporate the solvent to dryness, add 600ml of acetic acid, 36g of KI and 36g of sodium hypophosphite, reflux, react for 20 hours, cool down, filter, and water After rinsing, 17.49 g of a yellow product was obtained with a purity of 99.5% and a yield of 90%.
[0096] 2) Synthesis of compound 1
[0097] N 2 Under gas protection, 9,10-bis-(4-bromophenyl)anthracene 16.53...
Embodiment 2
[0110] Synthesis of (Compound 2)
[0111] The synthetic route is as follows:
[0112]
[0113] 1) Synthesis of compound 2-1
[0114] N 2 Under gas protection, in a 500mL three-necked flask, 9.58g (0.034mol) of 4-bromoiodobenzene, 7.21g (0.034mol) of 2-(3-fluorophenyl) benzimidazolyl, and 2.6g of copper iodide (purity AR0.0136mol), piperidinecarboxylic acid 5.4g (purity AR0.0136mol), potassium carbonate 35g (purity AR0.254mol). Use 600ml of DMF, magnetically stir, reflux for 30 hours, let cool and filter, and boil the crude yellow solid product several times with ethanol to obtain 9.96g of yellow solid with a purity of 99.6% and a yield of 80%.
[0115] 2) Synthesis of compound 2-2
[0116] Add 73.20g 4-(2-(3-fluorophenyl) benzimidazolyl)bromobenzene (compound 1-1, 0.2mol) and 1000ml tetrahydrofuran into a 2L dry and clean three-necked flask, protect it with nitrogen, and cool down to - 80°C, add 80ml of butyllithium dropwise, and react for 1 hour under temperature co...
Embodiment 3
[0120] Embodiment 3: the synthesis of compound 3
[0121]
[0122] N 2 Under air protection, 11.52 g of 6,12-dibromochryl (purity 98%, 0.03 mol), 24.91 g of 4-(2-(3-fluorophenyl) benzimidazolyl) phenylboronic acid were added to a 1000 mL three-necked flask (purity 99%, 0.075mol), tetrakistriphenylphosphine palladium 0.69g (purity AR, 0.0006mol), potassium carbonate 25.88g (purity AR, 0.188mol), toluene 300ml, ethanol 50ml, water 50ml. The above materials are heated to reflux. After 30 hours, the reaction was stopped, allowed to cool, and filtered to obtain a yellow solid, which was recrystallized from THF and repeated twice. 19.21 g of light yellow product was obtained with a purity of 99.80% and a yield of 80%.
[0123] Product MS (m / e): 800.28; Elemental analysis (C 56 h 34 f 2 N 4 ): theoretical value C: 83.98%, H: 4.28%, F: 4.74%, N: 7.00%; measured value C: 83.97%, H: 4.29%, F: 4.72%, N: 7.02%
[0124] According to the technical solutions of Example 2 and Examp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com