Blue light ionic type iridium complex as well as preparation method and application thereof
An iridium complex and ion-type technology, which is applied in the direction of indium organic compounds, platinum group organic compounds, chemical instruments and methods, etc., can solve the problems of material waste, achieve easy purification and separation, promote carrier transport, and have good solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The preparation method of the blue light ionic iridium complex of the present invention comprises the following steps:
[0051] Step 1, under the condition of inert gas nitrogen, in 2-ethoxyethanol solvent system, react difluorobipyridine derivatives with polyhydrated iridium trichloride in a molar ratio of 2.2:1 at 110-140°C to obtain iridium ( III) chlorine-bridged dimers;
[0052] Step 2, in the solvent system of dichloromethane and methanol, in the presence of anhydrous potassium carbonate, the iridium (III) chlorine bridge dimer and tetrabutylammonium cyanide, pyridine, 2,2'-bipyridine or 1 , One of the 10-phenanthrolines reacts at 30-60° C. in a molar ratio of 1:1.5-10 to obtain a blue-light iridium (III) complex.
[0053] Specifically, the synthetic route and synthetic steps are:
[0054]
[0055] Step 11, add compound 1, borate of D, and tetrakistriphenylphosphopalladium into a light-proof reaction bottle, and add tetrabutylammonium bromide, toluene, K 2 C...
Embodiment 1
[0062] Synthesis of dimer 4:
[0063]
[0064] The specific steps are implemented as follows:
[0065] Step 1: Mix 4-(2-ethylhexyloxy)-2,6-dimethylphenylboronate (7.2g, 19.98mmol), 2-chloro-4-bromopyridine (3.85g, 20.01mmol) , Tetraphenylphosphopalladium (0.693g, 0.61mmol) and TBAB (0.64g, 20mmol) are put into 250mL reaction bottle, add 60mL toluene and 30mL concentration successively after changing nitrogen and be the K of 2mol / L 2 CO 2 Add it into a 250mL reaction bottle, and react at 85°C for 24h. After the reaction, it is concentrated and purified by chromatographic column to obtain a light yellow liquid (4.75g, 68.7%), which is compound 2.
[0066] Step 2: Compound 2 (3.5g, 10.12mmol), 2,6-difluoropyridine-3-boronic acid (1.93g, 12.14mmol), tetrakistriphenylphosphopalladium (0.346g, 0.3mmol) obtained in step 1 Put it into a 250mL reaction bottle, add 30mL of toluene and 10mL of K with a concentration of 4mol / L in sequence after replacing nitrogen 2 CO 2 React with...
Embodiment 2
[0073] The synthetic steps of dimer 4 are the same as in Example 1;
[0074] Synthesis of D1Ir-Py:
[0075]
[0076] The specific steps are implemented as follows:
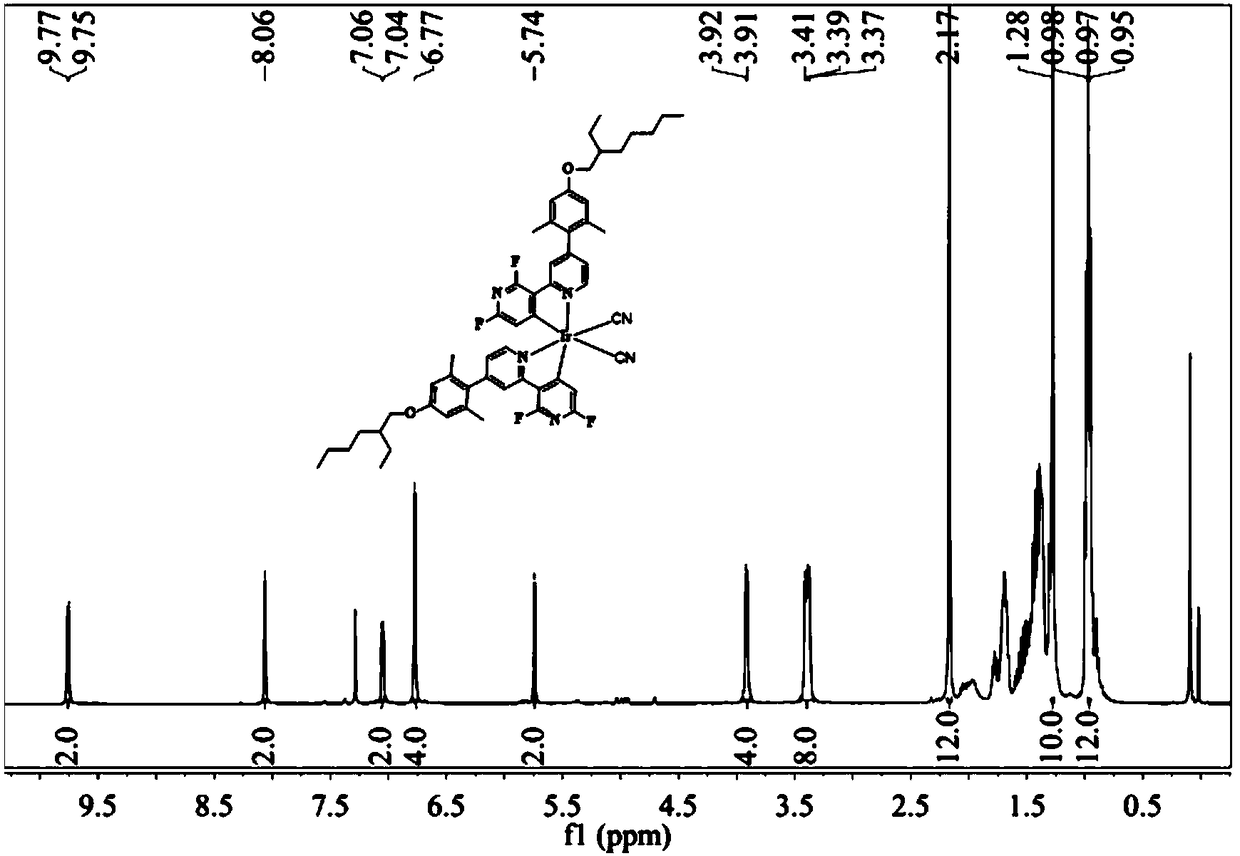
[0077] Reaction conditions: put dimer 4 (100mg, 0.048mmol) and pyridine (37.66mg, 0.48mmol) in a 50mL reaction tube, after pumping nitrogen, add 10mL dichloromethane and 5mL methanol into the reaction tube, and react at room temperature for 12h , after the reaction was completed, D1Ir-Py (43mg, 45.5%) was obtained by concentration and column purification. D1Ir-Py: 1 H NMR (400MHz, CDCl 3 ,δ):8.48(d,J=5.7Hz,2H),8.09(s,1H),7.16(dd,J=5.9,1.7Hz,1H),6.76(d,J=3.3Hz,2H),5.68 (s,1H),3.90(d,J=5.1Hz,2H),2.17(d,J=15.4Hz,6H),1.91(s,3H),1.75(dd,J=11.8,5.8Hz,1H) ,1.54–1.42(m,4H),1.40–1.31(m,5H),0.94(dd,J=13.0,7.3Hz,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com