Foam carbon-based catalyst and preparation method and application thereof
A carbon foam and catalyst technology, applied in the preparation of hydroxyl compounds, chemical instruments and methods, and hydrocarbon production from carbon oxides, can solve problems affecting product distribution, product secondary reactions, etc., and achieve excellent anti-coking and anti-oxidation performance, promotion of reduction, effect of not easy deactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A kind of preparation method of the foamed carbon base Fe catalyst that can be used for Fischer-Tropsch synthesis, the steps are as follows:
[0049] 1. Pretreatment of carbon foam material:
[0050] 1) Weigh 2.0g foam carbon (specific surface area is 10m 2 / g, containing macropores and mesopores) was added to 40mL of 68wt% nitric acid aqueous solution, stirred at 60°C after mixing, and heated to reflux for 2h to obtain a mixed solution;
[0051] 2) The above mixed solution was filtered, washed with deionized water to pH ≈ 7, and then dried at 60° C. for 10 h to obtain surface-treated carbon foam.
[0052] 2. Preparation of foamed carbon-based Fe catalyst:
[0053] Weigh 1.6g of ferric nitrate, 0.01g of potassium nitrate, 0.35g of manganese nitrate and 0.08g of copper nitrate, add 10mL of water after mixing to prepare a solution, impregnate the solution with 2.0g of surface-treated foam carbon, and pump Vacuum, and then put it in a drying oven to dry, the drying temp...
Embodiment 2
[0058] Same as Example 1, the difference is: in step 1, the method for pretreating the foamed carbon material is:
[0059] 1) Weigh 0.8g foam carbon (specific surface area is 200m 2 / g, containing micropores and mesopores) was added to 100mL34wt% nitric acid aqueous solution, stirred at 100°C after mixing, and heated to reflux for 20h to obtain a mixed solution;
[0060] 2) The above mixed solution was filtered, washed with deionized water to pH ≈ 7, and then dried at 150° C. for 20 h to obtain surface-treated carbon foam.
[0061] All the other are the same as step 2 and step 3 of embodiment 1, and the reaction results are shown in table 1.
Embodiment 3
[0063] Same as Example 1, the difference is: in step 2, the method for preparing the foamed carbon-based Fe catalyst is:
[0064] Weigh 1.6g of ferric nitrate and add 10mL of water to prepare a solution, impregnate the solution with 2.0g of surface-treated foam carbon, then vacuumize at room temperature, and then put it in a drying oven to dry at a temperature of 60°C and a constant temperature of 48h. And calcined at 800°C for 8h under Ar atmosphere to obtain Fischer-Tropsch synthesis foam carbon-based Fe catalyst.
[0065] Wherein, the loading amount of active metal in the obtained catalyst is about 10wt%.
[0066] All the other are the same as Step 1 and Step 3 of Example 1, and the reaction results are shown in Table 1.
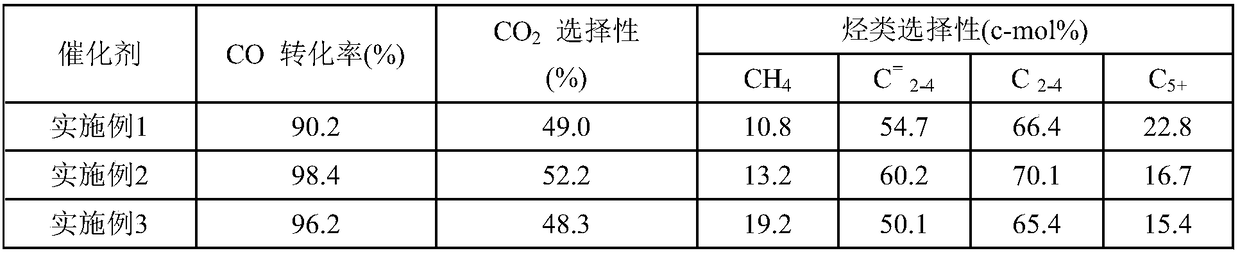
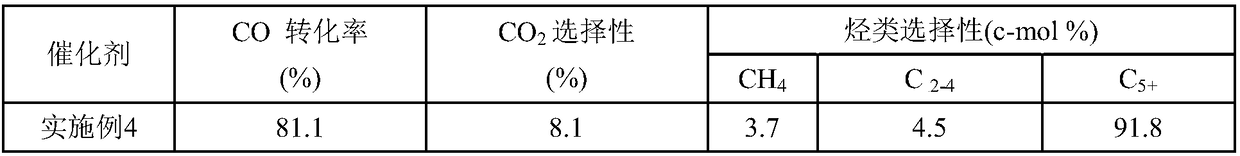
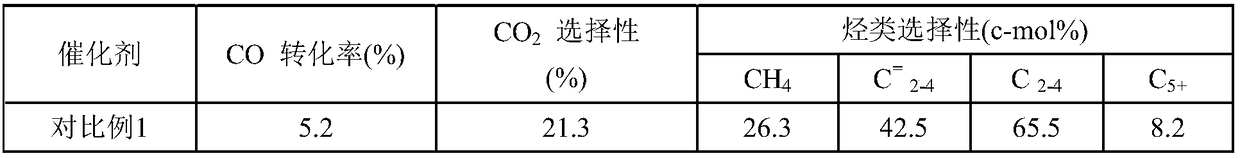
[0067] Table 1 Fischer-Tropsch synthesis catalyst reaction results
[0068]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com