Method for preparing 2,3-dichloropyridine through Sandmeyer reaction catalyst
A technology of dichloropyridine and catalyst, applied in the field of preparation of pyridine derivatives, can solve problems such as difficult separation, complex composition, difficulty in separating target products, etc., and achieve the effect of saving funds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
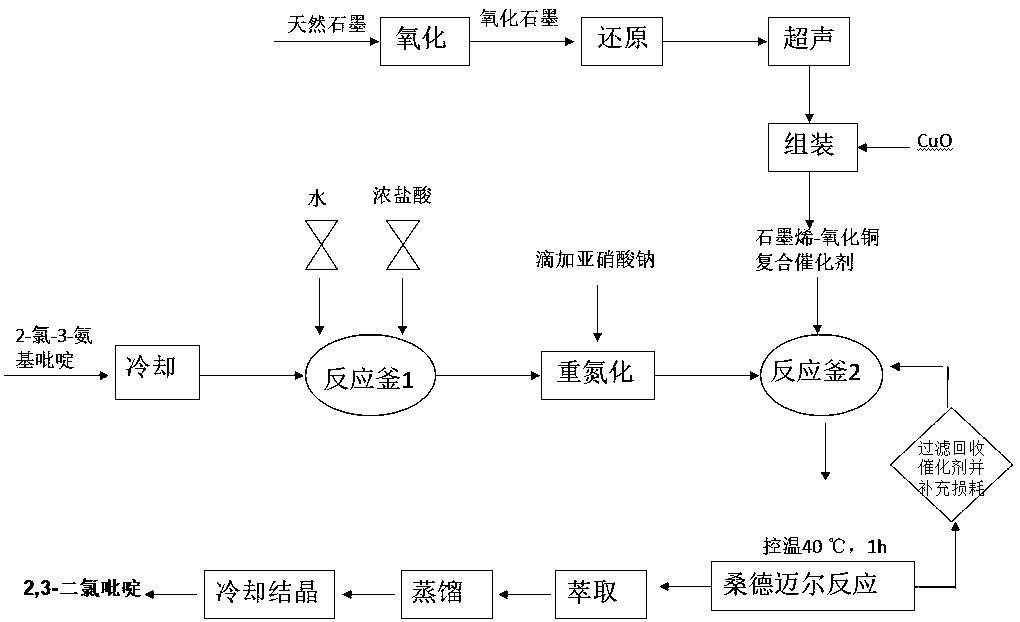
[0029] Embodiment 1: the synthesis of 2,3-dichloropyridine
[0030] The natural graphite was treated with concentrated sulfuric acid-potassium permanganate system to obtain graphite oxide, then ultrasonicated for 1 hour in the presence of hydrazine hydrate, and copper oxide was added to continue ultrasonic oscillation for 30 minutes to prepare a graphene-copper oxide composite catalyst. The mass fraction of copper in the composite catalyst is about 50-55%.
[0031] Put 400ml of industrial hydrochloric acid (3.5mol) in a 1000ml three-necked flask, cool down to -5°C with an ice-salt bath, turn on electromagnetic stirring, slowly add 129g (1mol) of 2-chloro-3-aminopyridine, and continue stirring to make 2-chloro -3-aminopyridine fully dissolved; then 70g NaNO 2 (1mol) dissolved in 150ml of water and slowly added dropwise to the above-mentioned three-neck flask, the temperature was controlled at 0~-5°C, after the dropwise addition was completed, keep warm at -5°C for use.
[003...
Embodiment 2
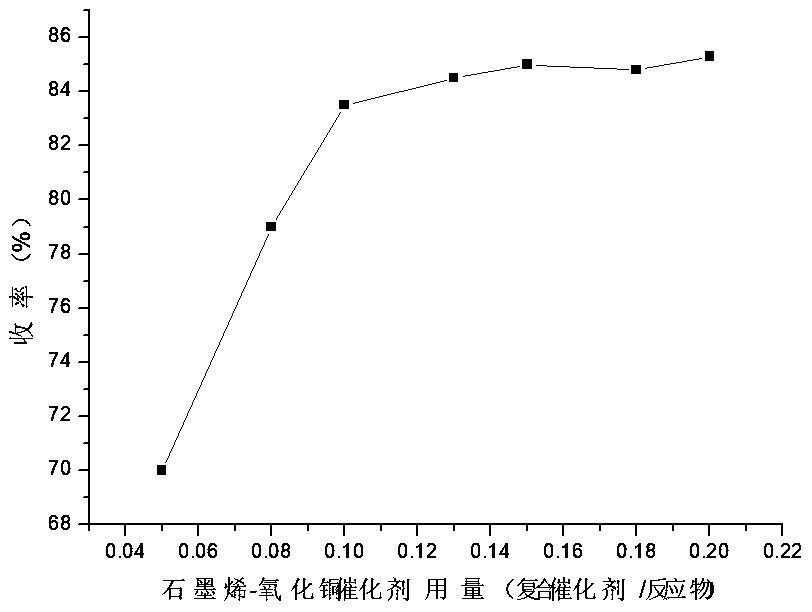
[0033] Embodiment 2: the impact of self-made catalyst consumption on reaction yield
[0034] In this example, the other reaction conditions were kept constant, and the influence of the amount of catalyst on the reaction yield was investigated.
[0035] The experimental results are attached figure 2 It can be seen that the yield of the reaction increases with the increase of the amount of catalyst. When the molar ratio of 2-chloro-3-aminopyridine to the catalyst reached 1:0.1, and then continued to increase the amount of the catalyst, the yield of the reaction remained basically unchanged. From the perspective of cost saving, we determined that the amount of catalyst used was 0.1 times that of 2-chloro-3-aminopyridine. Simultaneously, the results of parallel experiments show that the yield (80-85%) that can be achieved under this condition is slightly higher than that of the traditional process (using 0.2 times the equivalent of cuprous chloride catalyst, the yield is 78%). ...
Embodiment 3
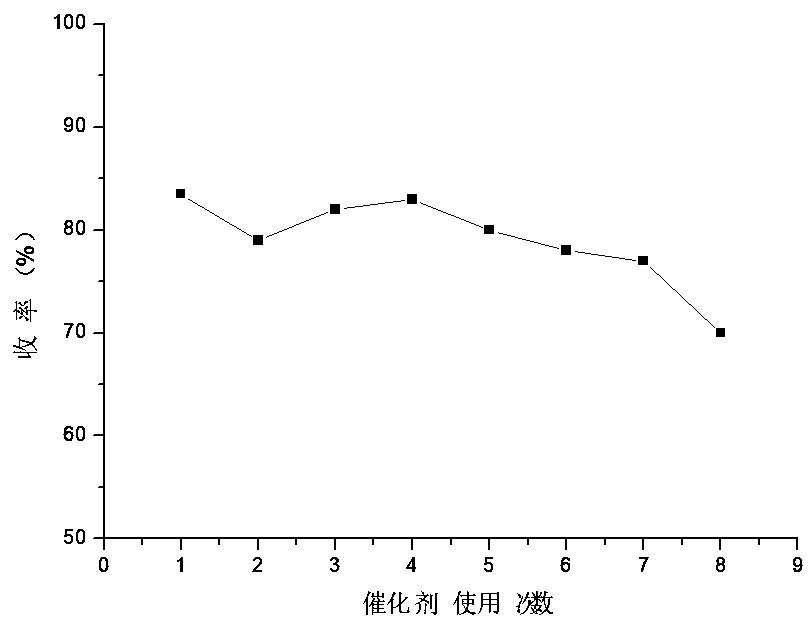
[0036] Embodiment 3: recovery and reuse of self-made catalyst
[0037] In the traditional production process, after the Sandmeyer reaction is completed, the target product is obtained by steam distillation or extraction separation, and the remaining residual liquid is discharged as three wastes, and a large amount of copper-containing wastewater is released in vain, which not only brings economic losses, but also It will cause environmental pollution, so the present invention explores the recovery and reuse of self-made composite catalysts. After the Sandmeyer reaction is completed, the pH value of the strongly acidic raffinate is adjusted to weakly acidic by using the alkaline waste liquid of the first step reaction , filter out the catalyst, and add part of the new catalyst according to the actual consumption for the next reaction. The fixed catalyst consumption is 0.1 times equivalent of the reactant, and the reaction is repeated 8 times, and the experimental results are as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com