Coding gene of heat-resistant type pullulanase as well as recombinant expression thereof and application thereof
A technology of pullulanase and coding gene, which is applied in application, genetic engineering, plant genetic improvement, etc., can solve the problems that there is no detailed report of pullulanase from Geobacillus stearothermophilus, and achieve good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Amplification of the pullulanase gene
[0029] 1.1 Strains and their cultivation
[0030] The pullulanase gene of the present invention is cloned from Geobacillus stearothermophilus.
[0031] Geobacillus stearothermophilus (DSMZ456) of the present invention can be purchased directly from the German Culture Collection of Microorganisms, the strain number is DSMZ 456, and its original source is extracted from sugar beet juice in Austria. Therefore, the Geobacillus stearothermophilus described in the present invention can be obtained through commercial means, and can also be obtained through field collection or other means.
[0032] Glycerol bacteria were inoculated into No. 1 medium, cultured at 55°C for 2 days, and the bacteria were collected for genome extraction.
[0033] 1.2 Genome Extraction
[0034] Refer to the instructions of the Generay Bacterial Genomic DNA Rapid Extraction Kit.
[0035] 1.3 Amplification of the pullulanase gene from Geobacillus stearothermo...
Embodiment 2
[0045] Construction and Expression of Escherichia coli Recombinant Expression Vector Containing Pullulanase
[0046] 2.1 Recombinant expression vector pET-28a-Plu GS build
[0047] A large number of pMD19-TSimple Vectors containing the pullulanase gene of Geobacillus stearothermophilus were cloned in Escherichia coli DH5α host bacteria, and the plasmid was extracted with the AxyPrep plasmid DNA extraction kit (operating steps refer to the instructions), and EcoRI and XhoI (purchased from Themo ) for double enzyme digestion, gel cutting to recover the pullulanase gene fragment, and then ligated with the Escherichia coli expression vector pET-28a that was also digested with EcoRI and XhoI at 16°C overnight, and transformed into Escherichia coli DH5α (purchased from Tiangen Biotechnology (Beijing) Co., Ltd.), positive clones were verified by PCR and sequenced; the sequencing results showed that no frameshift and other base mutations occurred, indicating that the constructed reco...
Embodiment 3
[0052] Pullulanase activity assay
[0053] 3.1 Standard curve drawing
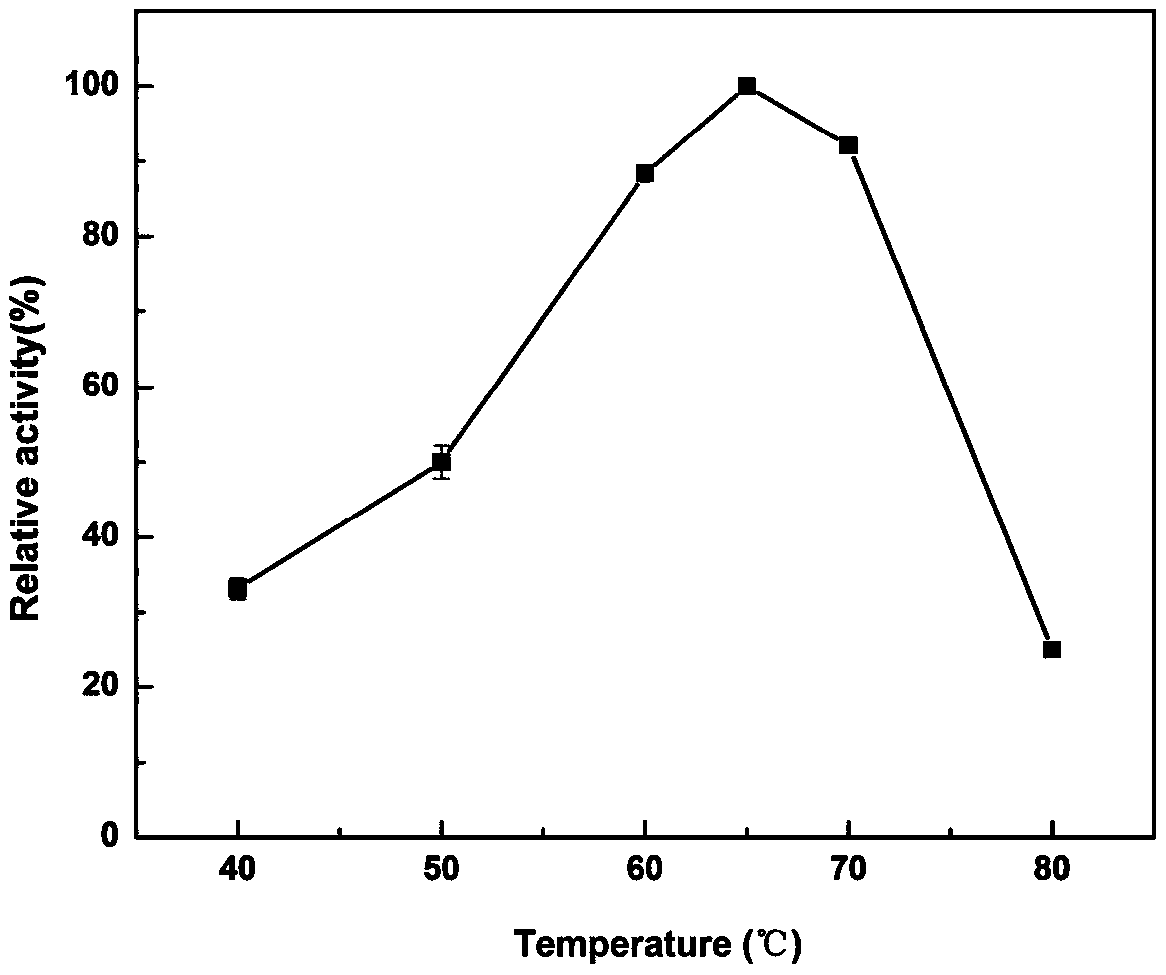
[0054] Take a clean test tube, label the test tube, prepare a glucose concentration gradient solution, add 0.2-1.4mL (at 0.2mL intervals) of 1% glucose solution to the test tube, and use the test tube without glucose as a blank control. Three parallel samples were made for each tube. Add ddH to the test tube respectively 2 O to a total volume of 2.0mL, then add 3mL DNS reagent to the test tube, boil for 15min, immediately add 10mL ddH 2 0 and pre-cooling, at a wavelength of 550nm, measure with a spectrophotometer colorimetrically, and write down the optical density value of the sample corresponding to each test tube to find its average value, then draw the glucose standard curve, and the results are shown in Figure 1.
[0055]3.2 Determination of enzyme activity
[0056] The enzymatic activity of recombinant pullulanase was measured by DNS termination method.
[0057] (1) Appropriately dilute the crud...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com