Method for comprehensively utilizing aluminum ash
A technology of aluminum ash and aluminum hydroxide, which is applied in the field of green recycling of solid waste, can solve the problems of long nitrogen removal time, no good process method, poor effect, etc., and achieve the effect of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
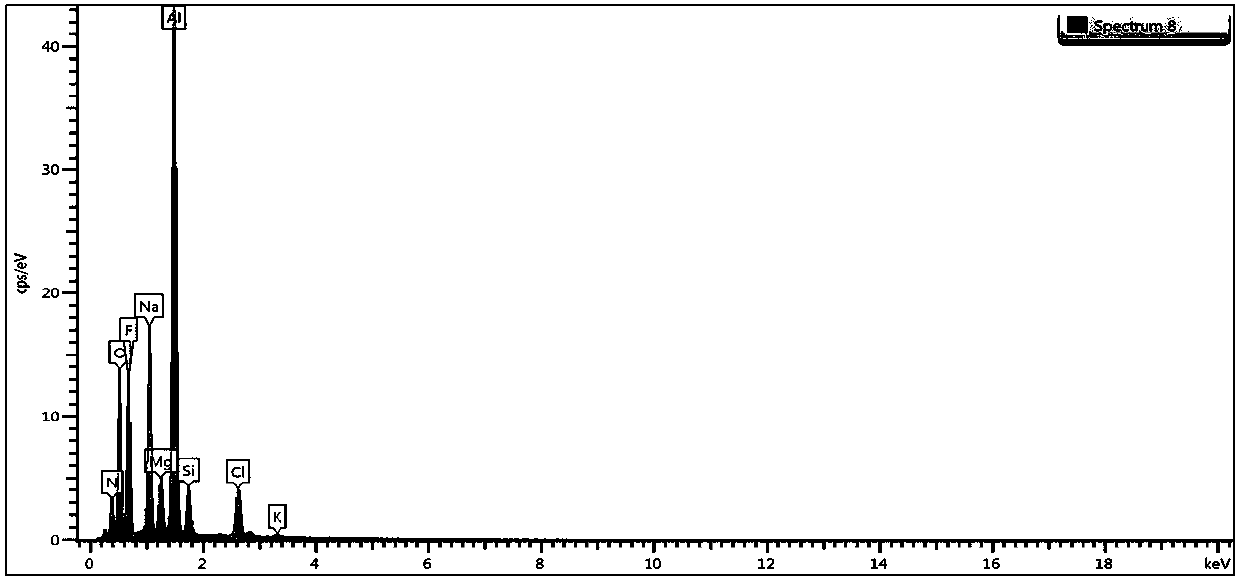
Image
Examples
Embodiment 1
[0043] After the aluminum ash is ground and passed through a 20-mesh sieve, it is mixed with ammonium bisulfate solution with a mass concentration of 30%, and the mixed slurry is heated to 200°C in a closed reaction kettle for reaction. The reaction time is 10 min. Ammonium bisulfate and aluminum ash The molar ratio of alumina in the medium is 4. During the heating reaction process, AlN is completely hydrolyzed, and the ammonia gas produced by AlN hydrolysis is completely absorbed by ammonium bisulfate to generate ammonium sulfate, thereby realizing zero discharge of ammonia gas. After the reaction, solid-liquid separation and washing are carried out to obtain a leaching solution with aluminum ammonium sulfate as the main component and a leaching residue with corundum as the main mineral composition to achieve 100% decomposition of aluminum nitride and ammonia gas absorption. Ammonia water or ammonia gas is passed into the obtained leaching solution for neutralization reaction...
Embodiment 2
[0045] The aluminum ash was ground and passed through a 20-mesh sieve and mixed with ammonium bisulfate solution with a mass concentration of 80%. The mixed slurry was heated to 70°C in a closed reaction kettle for reaction. The reaction time was 200 min. Ammonium bisulfate and aluminum ash The molar ratio of alumina in the medium is 16. During the heating reaction process, AlN is completely hydrolyzed, and the ammonia gas produced by AlN hydrolysis is completely absorbed by ammonium bisulfate to generate ammonium sulfate, thereby realizing zero discharge of ammonia gas. After the reaction, solid-liquid separation and washing are carried out to obtain a leaching solution with aluminum ammonium sulfate as the main component and a leaching residue with corundum as the main mineral composition to achieve 100% decomposition of aluminum nitride and ammonia gas absorption. Pass ammonia water or ammonia gas into the obtained leaching solution for neutralization reaction, solid-liquid...
Embodiment 3
[0047] The aluminum ash was ground and passed through a 20-mesh sieve and mixed with ammonium bisulfate solution with a mass concentration of 40%. The mixed slurry was heated to 180°C in a closed reaction kettle for reaction. The reaction time was 30 min. Ammonium bisulfate and aluminum ash The molar ratio of aluminum oxide in the medium is 8. During the heating reaction process, AlN is completely hydrolyzed, and the ammonia gas produced by AlN hydrolysis is completely absorbed by ammonium bisulfate to generate ammonium sulfate, thereby realizing zero discharge of ammonia gas. After the reaction, solid-liquid separation and washing are carried out to obtain a leaching solution with aluminum ammonium sulfate as the main component and a leaching residue with corundum as the main mineral composition to achieve 100% decomposition of aluminum nitride and ammonia gas absorption. Ammonia water or ammonia gas is passed into the obtained leaching solution for neutralization reaction, f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com