Preparation method of steviol
The technology of steviol aglycone and alcohol aglycone is applied in the field of preparation of steviol derivatives, which can solve the problems of affecting the reaction, extremely difficult separation and purification, unstable steviol and the like, and achieves the effect of simple reaction operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1R is the preparation of the steviol aglycone of TBDPS
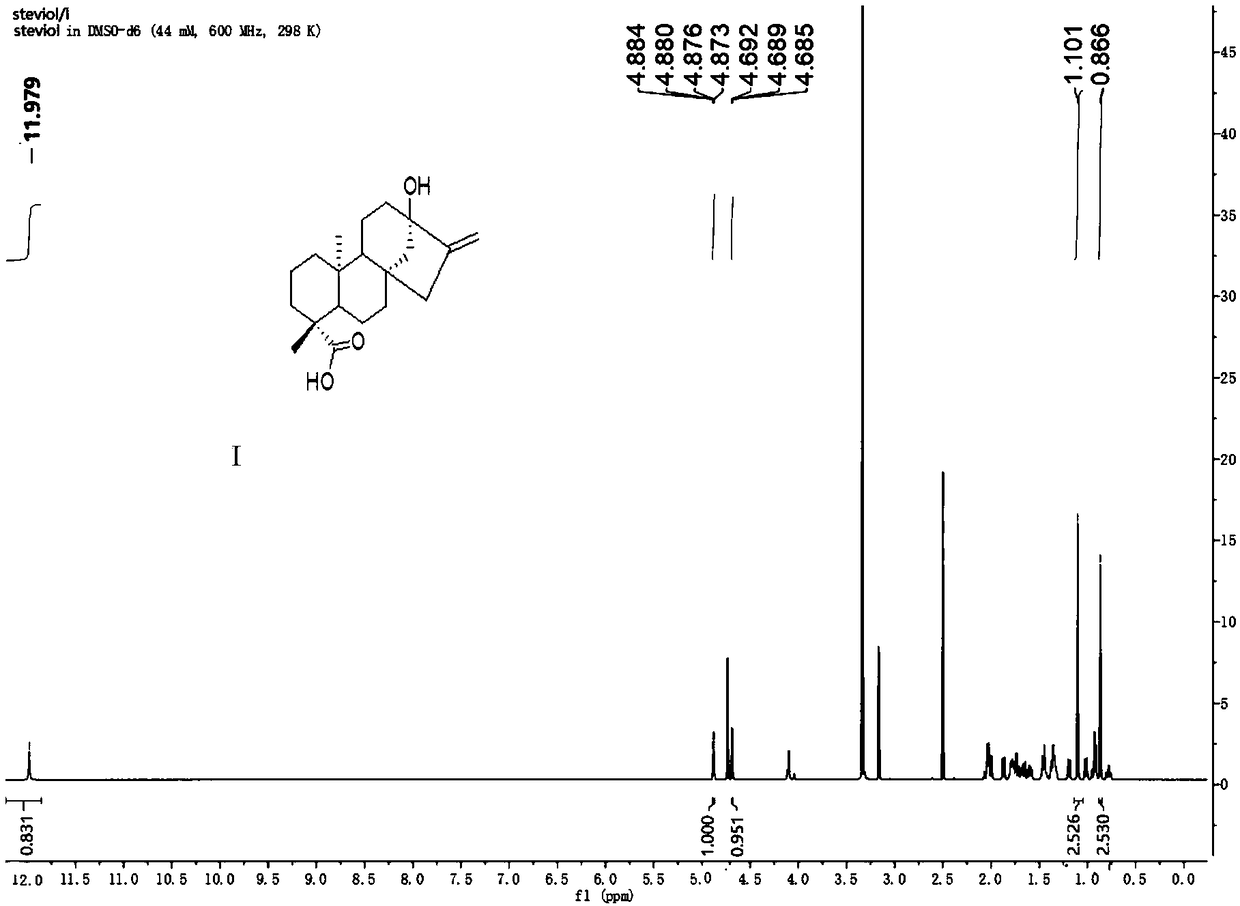
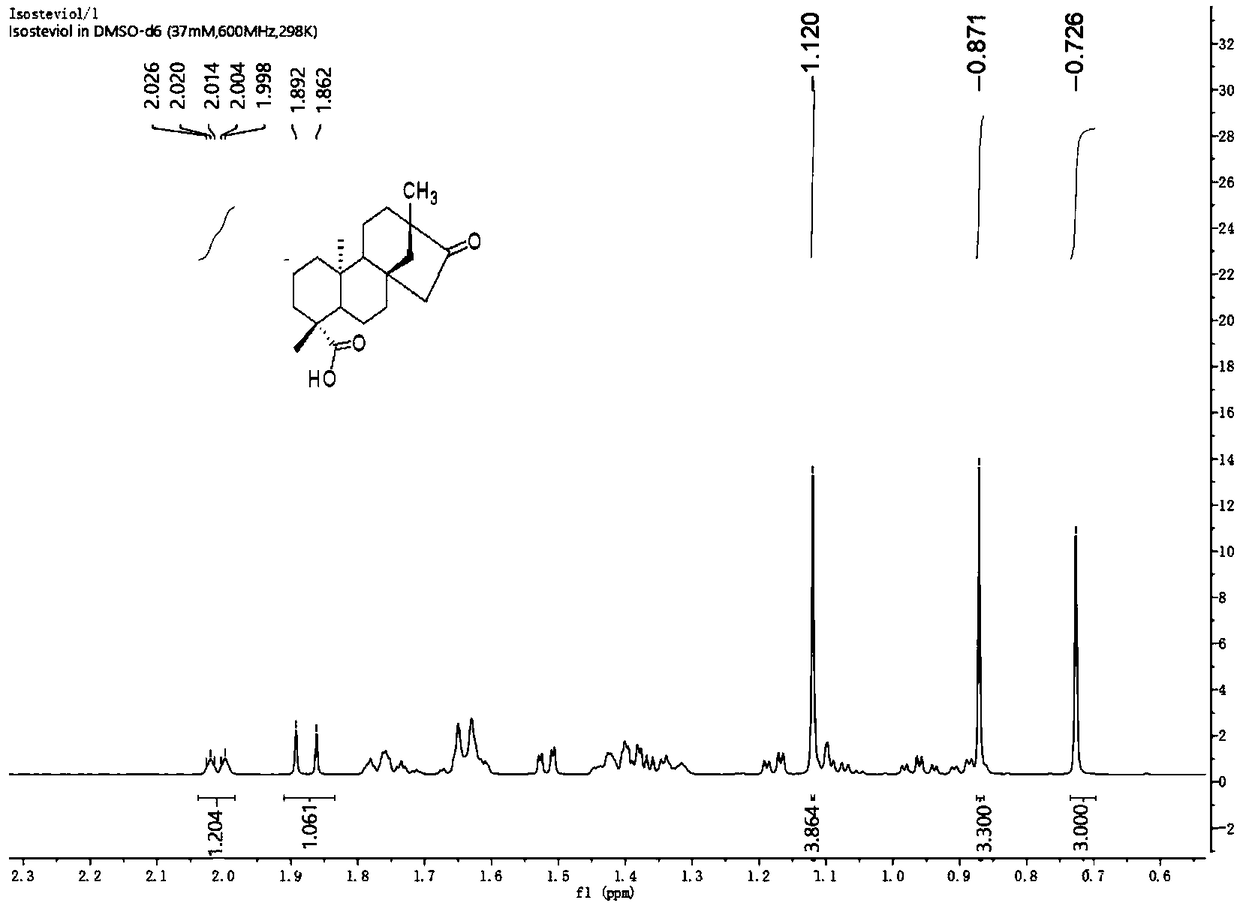
[0045] (1) Dissolve 100 g of stevia rebaudiana leaf crude extract powder in 250 mL of water, heat to dissolve, and slowly add 0.833 mL of concentrated hydrochloric acid with a concentration of 12 moL / L to the system, and reflux reaction at a stable 95 ° C for 28 hours. A white solid precipitated out. The reaction solution was completely cooled to room temperature and then filtered. The filter residue was dissolved in methanol at 60°C and recrystallized in a refrigerator at low temperature. After filtration, 12 g (0.0377 mol) of aglycone mixture was obtained as a white powdery solid.
[0046] (2) Dissolve 12 g (0.0377 mol) of the white powdery solid obtained in step (1) in 50 mL of N,N-dimethylformamide, add imidazole (1.5 eq) and TBDPSCl (1.5 eq), nitrogen React for 4 hours under protection, dilute with ethyl acetate, wash the organic phase three times with water, wash with saturated sodium chloride on...
Embodiment 2
[0051] Embodiment 2: R is the preparation of steviol aglycon of methylnaphthalene
[0052] (1) Dissolve 100 g of stevia leaf crude extract powder in 250 mL of water, heat to dissolve, then slowly add 0.41 mL of hydrochloric acid to the system, and reflux reaction at a stable 95°C for 28 hours, a white solid precipitates out. After cooling to room temperature, filter, take the filter residue and dissolve it in methanol at 60°C, place it in the refrigerator for low-temperature recrystallization, and obtain 12 g (0.0377 mol) of white powdery solid after filtration.
[0053] (2) Dissolve 5.6 g (0.0176 mol) of the white powdery solid obtained in step (1) in 25 mL of N,N-dimethylformamide, add imidazole (1.5 eq) and NapCl (1.5 eq), Under the protection of nitrogen, react for 4 hours, dilute with ethyl acetate, wash the organic phase three times with water, wash with saturated sodium chloride once, dry with anhydrous sodium sulfate, and spin the solvent to obtain 6.6 g (0.0144 mol) o...
Embodiment 3
[0058] Embodiment 3R is the preparation of the steviol aglycone of TBDPS
[0059] The preparation method is the same as in Example 1, except that in step (1), 100 g of stevia leaf crude extract powder is dissolved in 150 mL of water, and 0.625 mL of concentrated hydrochloric acid is slowly added dropwise; the temperature of the reflux reaction is 94 ° C, The time for the reflux reaction is 26 hours, and the solvent for the recrystallization is methanol. The molar ratio of steviol, isosteviol and double bond isomerization product in the obtained aglycon mixture is 1:1.2:1.2.
[0060] In step (2), the mass volume ratio of the aglycon mixture and the first organic solvent is 0.24g / mL, the base is triethylamine, and the molar ratio of the aglycone mixture, triethylamine and TBDPSCl is 1:1.2:1.2;
[0061] In step (3), the molar concentration of the aglycon mixture protected by the 19-position carboxyl group in DCM is 0.3mol / L, the epoxidation reagent is 30% hydrogen peroxide, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com