Heavy metal ion adsorbing material and preparation method thereof
A technology for heavy metal ions and adsorption materials, which is applied in the field of high-efficiency adsorption of heavy metal ions in wastewater and its preparation, can solve the problems of low equipment dependence, poor adsorption effect, and small adsorption capacity, and achieve low equipment dependence. Large, good adsorption effect, large adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
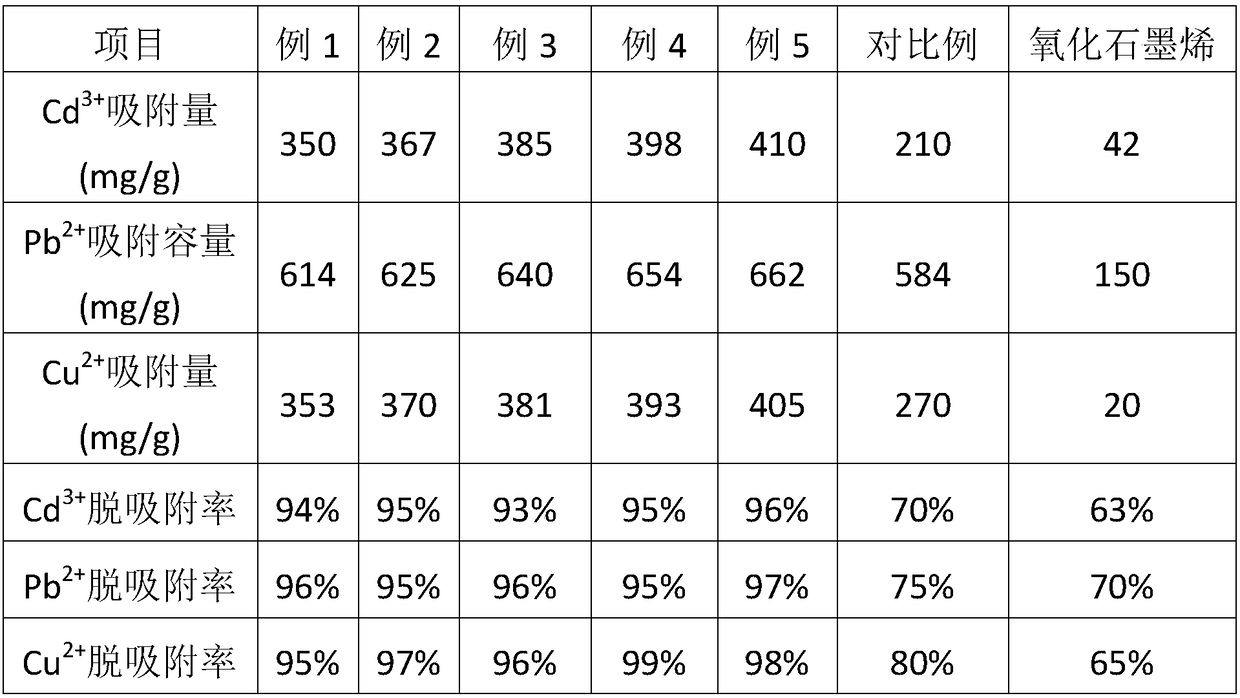
Embodiment 1
[0022] A heavy metal ion adsorption material, prepared from the following components by weight: 50 parts of cyclodextrin-based cucurbituril-based triazine copolymers, 20 parts of hyperbranched polyethyleneimine, 5 parts of graphene oxide share.
[0023] The preparation method of the cyclodextrin-based cucurbituril-based triazine copolymer comprises the following steps: 10 g of allyl cucurbituril, 10 g of allyl-β-cyclodextrin, 1,3-bis(ethylene oxide Alkylmethyl)-5-(2-propenyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione 30g, tert-butyl hydroperoxide 0.3g and Dissolve 0.4g of sodium dodecylbenzenesulfonate in 120g of dimethyl sulfoxide, stir and react under nitrogen atmosphere at 65°C for 3 hours, then settle out in water, filter, and bake in a vacuum oven at 100°C for 12 hours , to obtain cyclodextrin-based cucurbituril-based triazine copolymers.
[0024] The preparation method of the heavy metal ion adsorption material comprises the following steps: adding all raw materials into a...
Embodiment 2
[0026] A heavy metal ion adsorption material, prepared from the following components by weight: 53 parts of cyclodextrin-based cucurbituril-based triazine copolymers, 23 parts of hyperbranched polyethyleneimine, 6 parts of graphene oxide share.
[0027] The preparation method of the cyclodextrin-based cucurbituril-based triazine copolymer comprises the following steps: 10 g of allyl cucurbituril, 10 g of allyl-β-cyclodextrin, 1,3-bis(ethylene oxide Alkylmethyl)-5-(2-propenyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione 33g, dicumyl peroxide 0.35g and Dissolve 0.45g of polyoxypropylene polyvinylglycerol ether in 130g of N,N-dimethylformamide, stir and react at 69°C for 3.4 hours under a helium atmosphere, then settle out in water, filter, and then place in a vacuum drying oven 103 Bake at ℃ for 13 hours to obtain a cyclodextrin-based cucurbituril-based triazine copolymer.
[0028] The preparation method of the heavy metal ion adsorption material comprises the following steps: addin...
Embodiment 3
[0030] A heavy metal ion adsorption material, prepared from the following components by weight: 55 parts of cyclodextrin-based cucurbituril-based triazine copolymers, 26 parts of hyperbranched polyethyleneimine, 7 parts of graphene oxide share.
[0031] The preparation method of the cyclodextrin-based cucurbituril-based triazine copolymer comprises the following steps: 10 g of allyl cucurbituril, 10 g of allyl-β-cyclodextrin, 1,3-bis(ethylene oxide Alkylmethyl)-5-(2-propenyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione 36g, azobisisobutyronitrile 0.4g and Dissolve 0.5g of nonylphenol polyoxyethylene ether in 135g of N-methylpyrrolidone, stir and react at 70°C for 3.6 hours under a neon atmosphere, then settle out in water, filter, and dry in a vacuum oven at 105°C for 13.5 hours, a cyclodextrin-based cucurbituril-based triazine copolymer was obtained.
[0032] The preparation method of the heavy metal ion adsorption material comprises the following steps: adding all the raw materi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com